Filed Pursuant to Rule 424(b)(3)
Registration No. 333-115363
PROSPECTUS
$275,000,000
WH HOLDINGS (CAYMAN ISLANDS) LTD.
WH CAPITAL CORPORATION
Indirect Parent of
![]()
Exchange Offer for All Outstanding
91/2% Notes Due April 1, 2011
(CUSIP Nos. 92926X AA 3 and G96013 AA 8)
For New
91/2% Notes Due April 1, 2011
Which Have Been Registered
Under the Securities Act of 1933
This exchange offer will expire at 5:00 p.m., New York City time,
on Wednesday, July 9, 2004, unless extended.
We are offering to exchange our 91/2% Notes Due April 1, 2011 (New Notes) which have been registered under the Securities Act of 1933, as amended, for any and all of our outstanding 91/2% Notes Due April 1, 2011 (Outstanding Notes) in the aggregate principal amount of $275,000,000.
See the "Description of Notes" section for more information about the New Notes to be issued in this exchange offer.
The New Notes involve substantial risks similar to those associated with the Outstanding Notes. See the section entitled "Risk Factors" beginning on page 18 for a discussion of these risks.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES AND EXCHANGE COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR THE ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Prospectus dated June 9, 2004.
| |
Page |
|
|---|---|---|
| Disclosure Regarding Forward-Looking Statements | i | |
| Market Data | ii | |
| Prospectus Summary | 1 | |
| Risk Factors | 18 | |
| The Recapitalization of Holdings and Related Transactions | 28 | |
| The Exchange Offer | 29 | |
| Use of Proceeds | 39 | |
| Capitalization | 40 | |
| Selected Consolidated Historical Financial Data | 41 | |
| Unaudited Pro Forma Condensed Consolidated Financial Statements | 44 | |
| Management's Discussion and Analysis of Financial Condition and Results of Operations | 48 | |
| Business | 66 | |
| Management | 84 | |
| Executive Compensation | 87 | |
| Principal Shareholders | 101 | |
| Certain Relationships and Related Transactions | 105 | |
| Description of Other Indebtedness | 109 | |
| Description of Notes | 111 | |
| Form and Transfer of the Notes | 149 | |
| United States Federal Income Tax Consequences | 154 | |
| Certain ERISA Considerations | 158 | |
| Cayman Islands Tax Consequences | 160 | |
| Plan Of Distribution | 161 | |
| Legal Matters | 162 | |
| Experts | 162 | |
| Available Information | 162 | |
| Index to Consolidated Financial Statements | F-1 |
You should rely only on the information contained in this document or to which we have referred you. We have not authorized anyone to provide you with information that is different. This document may only be used where it is legal to sell these securities. The information in this document may only be accurate on the date of this document.
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains "forward-looking statements" within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). All statements other than statements of historical fact are "forward-looking statements" for purposes of federal and state securities laws, including any projections of earnings, revenue or other financial items; any statements of the plans, strategies and objectives of management for future operation; any statements concerning proposed new services or developments; any statements regarding future economic conditions or performance; any statements of belief; and any statements of assumptions underlying any of the foregoing. Forward-looking statements may include the words "may," "will," "estimate," "intend," "continue," "believe," "expect" or "anticipate" and other similar words.
Although we believe that the expectations reflected in any of our forward-looking statements are reasonable, actual results could differ materially from those projected or assumed in any of our forward-looking statements. Our future financial condition and results of operations, as well as any forward-looking statements, are subject to change and to inherent risks and uncertainties, such as those disclosed in this prospectus. Important factors that could cause our actual results, performance and achievements, or industry results to differ materially from estimates or projections contained in forward-looking statements include, among others, the following:
Additional factors that could cause actual results to differ materially from the forward-looking statements are set forth in this prospectus, including under the headings "Prospectus Summary," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Business" and in our "Prospectus Summary—Summary Historical Consolidated Financial Data" and the related notes. We do not intend, and undertake no obligation, to update any forward-looking statement.
This exchange offer involves risks. Before you tender your Outstanding Notes in exchange for New Notes, you should carefully consider the matters set forth under the heading "Risk Factors" and all other information contained in this prospectus. All subsequent written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by the cautionary statements.
i
Market data and other statistical information used throughout this prospectus are based on independent industry publications, government publications, and reports by market research firms or other published independent sources. Some data are also based on our good faith estimates, which are derived from our review of internal surveys, as well as the independent sources listed above. Although we believe that these sources are reliable, we have not independently verified the information and cannot guarantee its accuracy or completeness.
ii
The following summary should be read in conjunction with, and is qualified in its entirety by, the more detailed information and financial statements (including the accompanying notes) appearing elsewhere in this prospectus. Unless otherwise noted, the terms "we," "our," "us," "Company" and "Successor" refer to WH Holdings (Cayman Islands) Ltd. ("Holdings") and its subsidiaries, including WH Capital Corporation ("WH Capital Corp.") and Herbalife International, Inc. and its subsidiaries ("Herbalife") for periods subsequent to Herbalife's acquisition on July 31, 2002, and the terms "we," "us," "our," "Company" and "Predecessor" refer to Herbalife before the acquisition for periods through July 31, 2002. Holdings is a holding company, with substantially all of its assets consisting of the capital stock of its indirect, wholly-owned subsidiary, Herbalife. See "—Corporate Structure." The term "Outstanding Notes" refers to the 91/2% Notes due 2011 issued on March 8, 2004. The terms "New Notes" and "Notes" refer to the 91/2% Notes due 2011 offered by this prospectus. Unless the context indicates otherwise, "on a pro forma basis" or "pro forma" means after giving effect to the offering of the Notes and the transactions described under "Unaudited Pro Forma Condensed Consolidated Financial Data." "Adjusted EBITDA" is defined in Note 2 to the Summary Historical Consolidated Financial Data included herein. You should carefully consider the information set forth under "Risk Factors." In addition, certain statements are forward-looking statements which involve risks and uncertainties. See "Disclosure Regarding Forward-Looking Statements."
The Exchange Offer
In contemplation of the consummation of the Transactions described below under "—The Recapitalization of Holdings and Related Transactions," on March 8, 2004, Holdings and Capital completed a private offering of $275,000,000 of the Outstanding Notes. In connection with that offering, Holdings and Capital entered into a registration rights agreement with the initial purchaser of the Outstanding Notes in which they agreed, among other things, to deliver this prospectus and to complete this exchange offer within 195 days of the issue date of the Outstanding Notes. You are entitled to exchange in this exchange offer Outstanding Notes that you hold for New Notes with substantially identical terms. You should read the discussion under the headings "Summary of the Terms of the New Notes" beginning on page 13 and "Description of Notes" beginning on page 110 for further information regarding the New Notes.
We believe that the New Notes that will be issued in this exchange offer may be resold by you without compliance with the registration and prospectus delivery provisions of the Securities Act, if you can make the representations in the fifth paragraph under "The Exchange Offer—Exchange Terms" on page 28 below. We cannot guarantee that the Securities Exchange Commission, or SEC, would make a
1
similar decision about this exchange offer. If our belief is wrong, or if you cannot truthfully make the representations mentioned above, and you transfer any New Note issued to you in the exchange offer without meeting the registration and prospectus delivery requirements of the Securities Act, or without an exemption from such requirements, you could incur liability under the Securities Act. We are not indemnifying you for any such liability. You should read the discussion under the headings "Summary of the Terms of the Exchange Offer" beginning on page 10 and "The Exchange Offer" beginning on page 28 for further information regarding this exchange offer and resale of the New Notes.
The Company
Herbalife, founded in 1980, is a worldwide marketer of weight management, inner nutrition and Outer Nutrition® products that support our customers' wellness and healthy lifestyles. We market and sell these products through a global network marketing organization comprised of over one million independent distributors in 58 countries. Throughout our 24-year operating history, the high quality of our products and the effectiveness of our network marketing organization have been the primary drivers of our growth and geographic expansion and have made Herbalife a brand name recognized worldwide. For the year ended December 31, 2003, our net sales and adjusted EBITDA were approximately $1.2 billion and $167.7 million, respectively.
We are focused on delivering a science-based wellness program and way of life to our distributors and their customers through our product portfolio. Our products seek to appeal to the growing number of consumers who desire to live a healthy lifestyle. We group our products into three categories: weight management, which consists of a full range of meal replacement programs and healthy snacks; inner nutrition, which consists of nutritional supplements; and Outer Nutrition®, which represents personal care products. We currently market a broad range of products as well as literature and promotional materials designed to support our distributors' marketing efforts. Our products are often sold in programs, which are comprised of a series of related products designed to achieve a common wellness or health result, simplify weight management and nutrition for our consumers and maximize our distributors' cross-selling opportunities.
Weight management
Inner nutrition
2
43.6% of our net sales were derived from inner nutrition products. For the three months ended March 31, 2004, $139.3 million or 42.9% of our net sales were derived from inner nutrition products.
Outer Nutrition®
Our products are distributed through a global network marketing organization comprised of over one million independent distributors in 58 countries, except in China where our sales are regulated to be conducted on a wholesale basis to local retailers. We believe that the direct-selling distribution channel is ideally suited to selling our products, because sales of weight management and inner nutrition products are strengthened by ongoing personal contact between retail consumers and distributors. This personal contact enhances the education of consumers in the weight management and nutrition markets and the motivation of consumers to begin and maintain weight management programs for healthy living. In addition, our distributors use Herbalife's products themselves, providing first-hand testimonial proof of product effectiveness, which serves as a powerful sales tool.
We provide our distributors attractive and flexible career opportunities through selling our high-quality products. Our distributors have the opportunity to earn income and receive non-financial rewards designed to motivate and recognize individual achievement. As a result, we believe the income opportunity provided by our network marketing system appeals to a broad cross-section of people throughout the world, particularly those seeking to supplement family income, start a home business or pursue non-conventional, full and part-time employment opportunities. Our distributors, who are independent contractors, earn income on their own sales and can also earn royalties and bonuses on sales made by the distributors in their sales organizations. We believe our network marketing system provides an attractive income opportunity relative to other network marketing companies.
Holdings acquired Herbalife on July 31, 2002, which we refer to herein as the "Acquisition," and was formed by and on behalf of an investment group led by Whitney & Co., LLC ("Whitney") and Golden Gate Private Equity, Inc. ("Golden Gate"), which we refer to herein as the "Equity Sponsors," to consummate the Acquisition.
The Industry
Wellness and personal care markets
We compete primarily in the wellness industry with our weight management and inner nutrition products and in the personal care industry with our Outer Nutrition® products. According to Euromonitor, these markets are substantial in size, representing an estimated $34.8 billion and $173.0 billion of worldwide sales in 2001, respectively. We believe these markets represent significant business opportunities for us given our focus on high-quality products and our distributors' personal interaction with consumers of our products. According to Euromonitor:
3
We are capitalizing on demographic trends in our market driving demand for health-related products and increasing the emphasis on healthier lifestyles. The global markets for our products are expected to continue to experience strong growth due to a number of factors, including:
Direct selling
Direct selling as a distribution channel has proven to be extremely effective for sales of weight management, inner nutrition and Outer Nutrition® products and has exhibited strong historical growth. The World Federation of Direct Selling Associations estimates the following statistics about the direct selling channel:
Competitive Strengths
Recognized Brand Name. Our success is largely due to the strength of the Herbalife brand name, which has been built through consistently offering high-quality products and an attractive income opportunity for our distributors. Our specially-designed formulations and high-quality products, combined with our reputation built over 24 years in the industry, set us apart from the competition and contribute significantly to the recognition of the Herbalife brand name. We believe that our recognized brand name has contributed significantly to our ability to expand our business into new markets and add distributors to our network marketing organization and will enhance our ability to further penetrate our existing markets.
High-Quality, Established Product Portfolio. Our underlying initiative is to expand our reputation as a well-respected industry leader committed to providing the highest quality science-based products for our weight management and nutritional programs to support a healthier way of life. We are well positioned to take advantage of current trends concerning worldwide obesity and consumers increasingly turning to nutrition to address weight-related health concerns. In support of this effort, we have formed a Scientific Advisory Board comprised of leading worldwide experts to conduct product research and advise on product concepts. Members of this board include Chairman, David Heber,
4
M.D., Ph.D., Director of the UCLA Center for Human Nutrition and Director of the UCLA Center for Dietary Supplement Research in Botanicals, and Nobel Laureate in Medicine, Louis Ignarro, Ph.D., Distinguished Professor of Pharmacology at the UCLA School of Medicine. We recently established the Mark Hughes Cellular and Molecular Nutrition Laboratory at UCLA (the "UCLA Lab") through the donation of equipment and technology. Through the UCLA Lab, we fund diverse research projects that support both product development and marketing. We also have formed a Medical Advisory Board to provide training on product usage and give health-news updates through Herbalife literature, the internet, and live training events around the world. By drawing upon the technical resources of the UCLA Lab and combining its research with the scientific expertise of the Scientific Advisory Board, the educational skills of the Medical Advisory Board and our own research and development group, we will endeavor to enhance Herbalife's reputation as a provider of world class weight management and nutritional products that meet the highest industry standards for quality, safety and efficacy.
Effective Distribution Channel. We believe the direct sales model, the method we have used since we began operations in February 1980, is the most attractive and effective way to sell our products. Sales of our products are strengthened by ongoing personal contact between retail consumers and distributors, thereby enhancing the education of consumers in the weight management and nutrition markets. In turn, the motivation of consumers to begin and maintain weight management programs for healthy living is similarly enhanced. We also believe that sales of our weight management and inner nutrition products are strengthened by the first-hand, testimonial proof of product effectiveness provided by many of our distributors, who are consumers of our products themselves. Additionally, our global direct-selling model provides a more effective channel for our products than traditional retailing methods would: it eliminates a high, fixed-cost infrastructure, provides incentives for existing distributors to develop a sales organization of other individuals selling our products, and offers a highly attractive income opportunity to our distributors.
Product and Market Diversification. We offer a broad range of products across our three primary product segments. For the fiscal year ended December 31, 2003, 43.1% of our net sales were in weight management products, 43.6% in inner nutrition products, 9.1% in Outer Nutrition® products and the remaining 4.2% in literature and promotional materials. For the three months ended March 31, 2004, 43.8% of our net sales were in weight management products, 42.9% in inner nutrition products, 9.1% in Outer Nutrition® products and the remaining 4.2% in literature and promotional materials. Additionally, the geographic diversity of our markets mitigates our financial exposure to any particular market. For the fiscal year ended December 31, 2003, 36.6% of our net sales were in The Americas, 38.7% in Europe, 14.4% in Asia/Pacific Rim and 10.3% in Japan. For the three months ended March 31, 2004, 34.3% of our net sales were in The Americas, 42.2% in Europe, 14.2% in Asia/Pacific Rim and 9.3% in Japan.
Scalable and Profitable Business Model. We believe our business model is attractive due to our significant and sustained profitability and our ability to scale to new market segments and more distributors. We require no company-employed sales force to market and sell our products and our distributor compensation varies directly with sales. Lastly, our distributors bear the majority of our consumer marketing expenses, and supervisors sponsor and coordinate a large share of distributor recruiting and training initiatives.
Strong Cash Flow Generation in a Leveraged Operating Environment. Since the Acquisition, we have generated a significant amount of cash flow and reduced our net debt (total debt less cash and cash equivalents). At July 31, 2002, the date of the Acquisition, we had net debt of approximately $289.3 million. At December 31, 2003, we had reduced our net debt to approximately $168.9 million, an aggregate reduction of approximately $120.4 million over seventeen months. We currently expect our business will continue to generate strong cash flow, which we intend to use to service our debt and reduce indebtedness.
5
Experienced Management Team. Since the Acquisition, we have worked to strengthen our management team with experienced executives focused on making Herbalife the leading weight management and nutrition company worldwide. Since 2002, we have made several important additions to our management team that we believe will strengthen our capabilities in product development, operations and marketing. Our key hires are as follows:
Our management team also draws significant support from our many veteran members, including William D. Lowe (joined in 1998), our Senior Vice President, Principal Financial and Accounting Officer; Carol Hannah (joined in 1984), our President of Distributor Communications and Support and former Co-President; and Brian L. Kane (joined in 1993), our President of Herbalife's European region and former Co-President.
In addition, we recently extended an offer to Richard Goudis to serve as our new Chief Financial Officer for a three-year term commencing on June 14, 2004, which Mr. Goudis accepted on June 1, 2004.
Business Strategy
Our business strategy is comprised of the following principal elements:
Increase Brand Recognition with Science-based Weight Management and Nutrition Products. Since the Acquisition, we have significantly increased our emphasis on scientific research in the weight management and nutrition arena and increased our focus on developing products with scientifically demonstrated efficacy. Our underlying initiative is to continue to enhance our reputation as an industry leading and well-respected company committed to scientific research. For example, in 2003, we
6
introduced Niteworks™, a cardiovascular enhancement product developed by Dr. Louis Ignarro, a Nobel Laureate in Medicine. In addition, we recently funded and opened the Mark Hughes Lab for Cellular and Molecular Nutrition at UCLA. We have also established our own Scientific Advisory Board, which is committed to advancing the field of nutritional science. We are well positioned to take advantage of current worldwide consumer trends indicating that individuals are turning more and more towards weight management and nutritional products to address weight, fitness and age-related health concerns. We believe our focus on nutritional science will result in meaningful product enhancements and give consumers increased confidence in our products.
Provide Superior Personal Interaction, Care and Support for Our Customers. We believe the direct sales model, the method we have used since we began operations in February 1980, is the most attractive and effective way to sell our products in a manner that provides superior personal interaction, care and support for our customers. Sales of our products are strengthened by ongoing personal contact between retail consumers and distributors, thereby enhancing the education of consumers in the weight management and nutrition markets. In turn, the motivation of consumers to begin and maintain weight management programs for healthy living is similarly enhanced. We also believe that sales of our weight management and inner nutrition products are strengthened by the first-hand, testimonial proof of product effectiveness provided by many of our distributors, who are consumers of our products themselves.
Enhance the Visibility of Herbalife and Consumer Access to Herbalife Products. Our distributors are the only access point for consumers to purchase Herbalife products. While this distribution strategy is highly effective, we believe that we can assist our distributors in reaching a broader consumer base by increasing the visibility of Herbalife and its products. To accomplish this, we intend to supplement our distributors' selling efforts with Herbalife-sponsored marketing and public relations campaigns designed to generate consumer demand for our products. To allow consumers to access our products more easily, we are exploring implementing new ways for consumers to locate distributors, including internet-based referral systems and other new access points to Herbalife products.
Increase Market Penetration. Herbalife has historically grown principally by entering into new markets. Because Herbalife has already succeeded in entering into the most attractive markets for our products and distribution system, an increasingly important part of our strategy for continued growth is to increase the number and range of our products available in existing markets. We believe this will drive sales growth through increased penetration in each of our country markets around the world.
Increase Distributor Productivity and Retention. We recognize that in addition to high-quality products and a rewarding distributor compensation plan, the success of our business depends on the support and training of our distributors. We are strengthening our distributor support services by enhancing customer service capabilities at our call centers, offering greater business-building opportunities through the internet, creating new business support initiatives and offering enriched reward recognition programs. To further enhance distributor productivity and improve retention, we are developing new educational training programs aimed at assisting distributors with their sales, marketing and recruiting techniques. Extensive training opportunities enable distributors to not only develop invaluable business-building and leadership skills, but also to become experts in our products and offer customers sound advice on weight management, nutrition and personal care. By placing a top priority on training, we build credibility among our distributors and further establish Herbalife as an industry leader.
Improve Margins through Expense Management. During the last two years, we have implemented certain product manufacturing and other sustainable expense reduction initiatives that have already resulted in significant improvements in financial performance. We are focused on realizing savings through cost control of corporate expenses and continued focus on the implementation of the above initiatives. Through these initiatives and our improvement in net sales, we have improved our EBITDA from $86.8 million (8.5% of net sales) in 2001 to $167.7 million (14.5% of net sales) in 2003. We
7
believe the combination of reduced product costs and our continued control of corporate spending will enhance profitability and cash flow.
The Recapitalization of Holdings and Related Transactions
The proceeds of the offering of the Outstanding Notes, together with available cash, was used as a part of a recapitalization of Holdings, which consisted of (i) the redemption of all of Holdings' outstanding 12% Series A Cumulative Convertible Preferred Shares, which we refer to herein as the "Preferred Shares," including shares issued upon the exercise of warrants to purchase the Preferred Shares, which we refer to herein as the "Warrants;" (ii) the purchase of all of Holdings' outstanding 15.5% Senior Notes due 2011, which we refer to herein as the "Senior Notes;" (iii) the reduction of outstanding amounts under Herbalife's senior credit facilities; and (iv) the payment of related fees and expenses (collectively, the "Transactions").
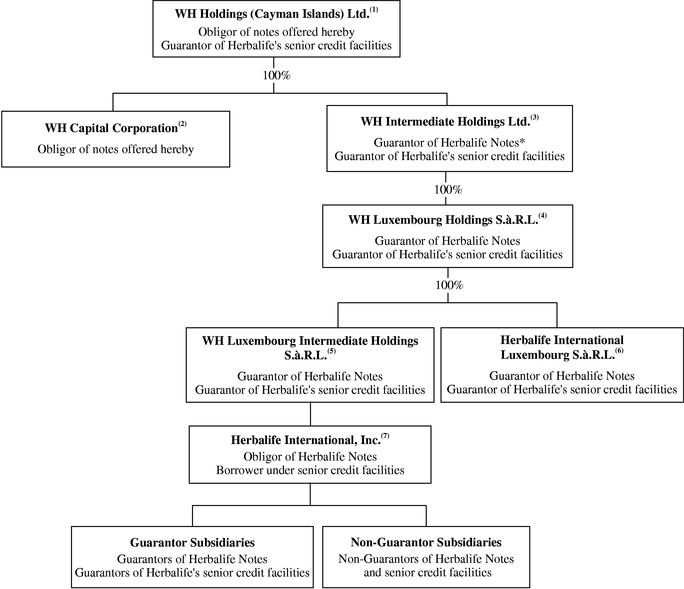
Corporate Structure
International activities are an important part of our business. In 2003, international sales accounted for approximately 76% of our net sales. Our ultimate parent company is organized in the Cayman Islands, and its immediate subsidiaries are organized either in the Cayman Islands or in Luxembourg in accordance with our foreign holding and operating company structure. We believe that this foreign holding and operating company structure provides us with an effective platform to organize our international business activities and to take advantage of favorable environments to implement our international business, operating and financial strategies.
8
The chart below illustrates our organizational structure after giving effect to the Transactions:
(footnotes on following page)
9
(footnotes from preceding page)
10
Summary of the Terms of the Exchange Offer
The following is a summary of the principal terms of the exchange offer. A more detailed description is contained in the section "The Exchange Offer." The term "Outstanding Notes" refers to our outstanding 91/2% Notes due 2011, and the terms "New Notes" and "Notes" refer to our 91/2% due 2011. The term "indenture" refers to the indenture that governs both the Outstanding Notes and the New Notes.
| The Exchange Offer | We are offering to issue registered New Notes in exchange for a like principal amount and like denomination of our Outstanding Notes. We are offering to issue these registered New Notes to satisfy our obligations under a registration rights agreement that we entered into with the initial purchaser of the Outstanding Notes when we sold the Outstanding Notes in a transaction that was exempt from the registration requirements of the Securities Act. The terms of the New Notes offered in the exchange offer are substantially identical to those of the Outstanding Notes. You may tender your Outstanding Notes for exchange by following the procedures described under the caption "The Exchange Offer." | |||
Registration Rights Agreement |
We sold the Outstanding Notes on March 8, 2004 to the initial purchaser in a transaction that was exempt from the registration requirements of the Securities Act. In connection with the sale, we entered into a registration rights agreement with the initial purchaser which grants the holders of the Outstanding Notes specified exchange and registration rights. This exchange offer satisfies those rights, which terminate upon consummation of the exchange offer. You will not be entitled to any exchange or registration rights with respect to the New Notes. |
|||
Expiration Date |
The exchange offer will expire at 5:00 p.m., New York City time, on July 9, 2004, which is 30 days after the commencement of the exchange offer, unless we extend it. |
|||
Withdrawal |
You may withdraw the tender of any Outstanding Notes pursuant to the exchange offer at any time prior to the Expiration Date. We will return, as promptly as practicable after the expiration or termination of the exchange offer, any Outstanding Notes not accepted for exchange for any reason without expense to you. |
|||
Interest on the Notes |
Interest on the New Notes will accrue from the date of the original issuance of the Outstanding Notes or from the date of the last payment of interest on the Outstanding Notes, whichever is later. No additional interest will be paid on Outstanding Notes tendered and accepted for exchange. |
|||
Conditions to the Exchange Offer |
The exchange offer is subject to customary conditions, some of which we may waive. |
|||
11
Procedures for Tendering Outstanding Notes |
If you wish to accept the exchange offer, you must complete, sign and date the accompanying letter of transmittal in accordance with the instructions in the letter of transmittal, and deliver the letter of transmittal, along with the Outstanding Notes and any other required documentation, to the exchange agent. |
|||
By executing the letter of transmittal, you will represent to us that, among other things: |
||||
• |
any New Notes that you receive will be acquired in the ordinary course of your business; |
|||
• |
you are not participating, and you have no arrangement or understanding with any person to participate, in the distribution of the New Notes; |
|||
• |
you are not our "affiliate", as defined in Rule 405 of the Securities Act or a broker-dealer tendering Outstanding Notes acquired directly from us; and |
|||
• |
if you are not a broker-dealer, you will also be representing that you are not engaged in and do not intend to engage in a distribution of the New Notes. |
|||
Each broker-dealer receiving New Notes for its own account in exchange for Outstanding Notes must acknowledge that it will deliver a prospectus meeting the requirements of the Securities Act in connection with any resale of the New Notes. The letter of transmittal states that, by making this acknowledgement and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an "underwriter" within the meaning of the Securities Act. A broker-dealer who acquired the Outstanding Notes for its own account as a result of market-making or other trading activities may use this prospectus for an offer to resell, resale or other transfer of the New Notes. |
||||
We will accept for exchange any and all Outstanding Notes which are properly tendered (and not withdrawn) in the exchange offer prior to the Expiration Date. The New Notes issued pursuant to the exchange offer will be delivered promptly following the Expiration Date. See "The Exchange Offer—Acceptance of Outstanding Notes for Exchange" beginning on page 30 of this prospectus. |
||||
Special Procedures for Beneficial Owners |
If you are a beneficial owner whose Outstanding Notes are registered in the name of a broker, dealer, commercial bank, trust company or other nominee and you wish to tender such Outstanding Notes in the exchange offer, please contact the registered holder as soon as possible and instruct them to tender your Outstanding Notes on your behalf and comply with our instructions set forth elsewhere in this prospectus. |
|||
12
Guaranteed Delivery Procedures |
If you cannot meet the Expiration Date deadline, or you cannot deliver your Outstanding Notes, the letter of transmittal or any other documentation on time, then you must surrender your Outstanding Notes according to the guaranteed delivery procedures set forth under "The Exchange Offer—Procedures for Tendering Outstanding Notes—Guaranteed Delivery" beginning on page 33 of this prospectus. |
|||
Acceptance of Outstanding Notes and Delivery of New Notes |
Upon consummation of the exchange offer, we will accept any and all Outstanding Notes that are properly tendered in the exchange offer and not withdrawn prior to 5:00 p.m., New York City time, on the Expiration Date. The New Notes issued pursuant to the exchange offer will be delivered promptly after acceptance of the tendered Outstanding Notes. |
|||
U.S. Federal Income Tax Considerations |
Your exchange of Outstanding Notes for New Notes to be issued in the exchange offer will not result in any gain or loss to you for U.S. federal income tax purposes. |
|||
Use of Proceeds |
We will not receive any cash proceeds from the exchange offer. |
|||
Exchange Agent |
The Bank of New York. |
|||
Consequences of Failure to Exchange your Outstanding Notes |
Outstanding Notes that are not tendered, or that are tendered but not accepted, will continue to be subject to the restrictions on transfer that are described in the legend on those notes. In general, you may offer or sell your Outstanding Notes only if they are registered under, or offered or sold under an exemption from, the Securities Act and applicable state securities laws. We, however, will have no further obligation to register the Outstanding Notes. If you do not participate in the exchange offer, the liquidity of your Outstanding Notes could be adversely affected. |
|||
Consequences of Exchanging Your Outstanding Notes |
Based on interpretations of the staff of the SEC, we believe that you may offer for resale, resell or otherwise transfer the New Notes that we issue in the exchange offer without complying with the registration and prospectus delivery requirements of the Securities Act if you make the representations that appear above under the heading "—Procedures for Tendering Outstanding Notes." |
|||
If any of these conditions is not satisfied and you transfer any New Notes issued to you in the exchange offer without delivering a proper prospectus or without qualifying for a registration exemption, you may incur liability under the Securities Act. We will not be responsible for or indemnify you against any liability you may incur. |
||||
13
Summary of the Terms of the Notes
The terms of the New Notes we are issuing in this exchange offer and the Outstanding Notes are identical in all material respects, except the New Notes offered in the exchange offer:
A brief description of the material terms of the Notes follows:
| Issuers | WH Holdings (Cayman Islands) Ltd., a Cayman Islands exempted limited liability company and its wholly-owned subsidiary WH Capital Corporation, a Nevada corporation. | |
Securities Offered |
$275,000,000 aggregate principal amount of 91/2% Notes due 2011. |
|
Maturity |
April 1, 2011. |
|
Interest |
The Notes will bear interest at the rate of 91/2% per year from March 8, 2004, payable semi-annually, in arrear, on April 1 and October 1 of each year, commencing on October 1, 2004. |
|
Ranking |
The Notes will be our general unsecured obligations, ranking: |
|
• equally with any of our existing and future senior indebtedness (other than Holdings' guarantee of Herbalife's obligations under its senior secured credit facilities, to which the Notes offered hereby will be contractually subordinated); and |
||
• senior to all of our future subordinated indebtedness. |
||
The Notes will be effectively subordinated to all existing and future indebtedness and other liabilities of our subsidiaries. |
||
As of March 31, 2004, our subsidiaries had total indebtedness of $243.2 million to which the Notes will be effectively subordinated in right of payment and that, among other things, limit our ability to access the value or cash flow of our subsidiaries. |
||
Guarantees |
Generally, our obligations under the Notes will not be guaranteed by any of our subsidiaries. Under certain circumstances, however, our subsidiaries may be required to guarantee our obligations under the Notes. See "Description of Notes—Limitations on Indebtedness." |
|
Optional Redemption |
We may redeem some or all of the Notes at any time and from time to time on or after April 1, 2008, at a redemption price equal to 100% of the principal amount plus a premium declining ratably to par, plus accrued and unpaid interest and liquidated damages, if any, to the redemption date. |
|
14
At any time prior to April 1, 2007, we may use the proceeds of certain equity offerings to redeem up to 40% of the aggregate principal amount of Notes originally issued under the indenture and all or a portion of any additional Notes issued after the date of the indenture, in each case at a redemption price equal to 109.50% of the principal amount, plus accrued and unpaid interest and liquidated damages, if any, to the redemption date. |
||
In addition, at any time and from time to time prior to April 1, 2008, we may redeem some or all of the Notes at a redemption price equal to 100% of the principal amount plus a make-whole premium, plus accrued and unpaid interest and liquidated damages, if any, to the redemption date. |
||
Change of Control |
Upon the occurrence of a change of control, we may be required to purchase the Notes at 101% of their principal amount plus accrued and unpaid interest, if any, to the purchase date. |
|
Certain Covenants |
The indenture governing the Notes contains covenants that will limit our and our subsidiaries' ability to, among other things: |
|
• pay dividends, redeem share capital or capital stock and make other restricted payments and investments; |
||
• incur additional debt or issue preferred shares; |
||
• allow the imposition of dividend or other distribution restrictions on our subsidiaries; |
||
• create liens on assets; |
||
• engage in transactions with affiliates; |
||
• guarantee other indebtedness of Holdings; and |
||
• merge, consolidate or sell all or substantially all of our assets and the assets of our subsidiaries. |
Federal Income Tax Consequences
See "United States Federal Income Tax Consequences" starting on page 153 of this prospectus.
Additional Information
Herbalife's principal executive offices are located at 1800 Century Park East, Los Angeles, California 90067. Herbalife's telephone number is (310) 410-9600.
The principal executive offices of Holdings and its subsidiaries are located c/o Whitney at 177 Broad Street, Stamford, Connecticut 06901. Their telephone number is (203) 973-1400.
Risk Factors
This investment involves risks. Before deciding to surrender your Outstanding Notes for New Notes pursuant to this exchange offer, you should carefully consider the matters set forth under the heading "Risk Factors" beginning on page 17 of this prospectus and all other information contained in this prospectus.
15
SUMMARY HISTORICAL CONSOLIDATED FINANCIAL DATA
The following table sets forth certain of our historical financial data. We have derived the summary historical consolidated financial data as of December 31, 2002 and 2003 and for the year ended December 31, 2001, the seven month period ended July 31, 2002, the five month period ended December 31, 2002, and the year ended December 31, 2003, from our audited financial statements and the related notes included elsewhere in this prospectus. We have derived the summary historical consolidated financial data for the three months ended March 31, 2003 and as of and for the three months ended March 31, 2004 from the unaudited financial statements and related notes included elsewhere in this prospectus. The summary historical financial data set forth below are not necessarily indicative of the results of future operations and should be read in conjunction with our audited consolidated financial statements, the selected consolidated historical financial data and the unaudited financial statements and, in each case, the related notes included elsewhere in this prospectus, the discussion under the heading "Management's Discussion and Analysis of Financial Condition and Results of Operations," and the historical consolidated financial statements and accompanying notes included elsewhere in this prospectus.
We present EBITDA and adjusted EBITDA because management believes it provides useful information regarding our ability to service and/or incur debt and that it provides a more comparable measure of our profitability. However, such a measure is not in accordance with GAAP. You should not consider EBITDA in isolation from or as a substitute for net income, cash flows from operating activities and other consolidated income or cash flow statement data prepared in accordance with accounting principles generally accepted in the United States or as a measure of profitability or liquidity. Adjusted EBITDA is calculated by adding back to EBITDA buy-out transaction expenses and other non-recurring expenses relating to the Acquisition. Adjusted EBITDA, as presented, may not be comparable to similarly titled measures reported by other companies.
For purposes of the presentation below, the pre-Acquisition and post-Acquisition periods for 2002 have been combined in order to facilitate comparisons. The combined information is not necessarily indicative of what actual results would have been for the year ended December 31, 2002.
| |
Historical |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, 2001 |
January 1 to July 31, 2002 |
August 1 to December 31, 2002 |
Year ended December 31, 2002 |
Year ended December 31, 2003 |
Three Months Ended March 31, 2003 |
Three Months Ended March 31, 2004 |
|||||||||||||||
| |
(dollars in thousands) |
|||||||||||||||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(predecessor and successor combined) |
(successor) |
(successor) |
(successor) |
|||||||||||||||
| Operations: | ||||||||||||||||||||||
| Net sales | $ | 1,020,130 | $ | 644,188 | $ | 449,524 | $ | 1,093,712 | $ | 1,159,433 | $ | 280,039 | $ | 324,053 | ||||||||
| Gross profit | 778,608 | 503,635 | 354,523 | 858,158 | 923,648 | 223,079 | 260,435 | |||||||||||||||
| Operating income(1) | 68,775 | 14,304 | 52,889 | 67,193 | 107,036 | 39,192 | 36,736 | |||||||||||||||
| Net income (loss) | 42,588 | 9,212 | 14,005 | 23,217 | 36,847 | 16,870 | (485 | ) | ||||||||||||||
Other Financial Data: |
||||||||||||||||||||||
| EBITDA(2) | 86,831 | 26,026 | 64,313 | 90,339 | 162,641 | 45,581 | 48,142 | |||||||||||||||
| Adjusted EBITDA(2) | $ | 86,831 | $ | 80,734 | $ | 70,496 | $ | 151,230 | $ | 167,733 | 45,923 | 48,142 | ||||||||||
| Adjusted EBITDA margin(3) | 8.5 | % | 12.5 | % | 15.7 | % | 13.8 | % | 14.5 | % | 16.4 | % | 14.9 | % | ||||||||
| Depreciation and amortization | $ | 18,056 | $ | 11,722 | $ | 11,424 | $ | 23,146 | $ | 55,605 | 6,389 | 11,406 | ||||||||||
| Capital expenditures(4) | 14,751 | 6,799 | 3,599 | 10,398 | 20,435 | 2,720 | 5,422 | |||||||||||||||
| Ratio of earnings to fixed charges(5) | 8.1 | 3.6 | 2.0 | 2.3 | 2.2 | 3.2 | 1.4 | |||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||||
| Cash, cash equivalents and marketable securities(6) | $ | 201,181 | $ | 76,024 | $ | 156,380 | $ | 123,002 | ||||||||||||||
| Receivables, net | 27,609 | 29,026 | 31,977 | 33,775 | ||||||||||||||||||
| Inventories | 72,208 | 56,868 | 59,397 | 64,134 | ||||||||||||||||||
| Total working capital(7) | 177,813 | 7,186 | 1,521 | 18,789 | ||||||||||||||||||
| Total assets | 470,335 | 855,705 | 903,964 | 873,016 | ||||||||||||||||||
| Total debt | 10,612 | 340,759 | 325,294 | 510,622 | ||||||||||||||||||
| Shareholders' equity | 260,916 | 191,274 | 237,788 | 16,776 | ||||||||||||||||||
16
The following table represents a reconciliation of net income to EBITDA and adjusted EBITDA:
| |
Historical |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, 2001 |
January 1 to July 31, 2002 |
August 1 to December 31, 2002 |
Year ended December 31, 2002 |
Year ended December 31, 2003 |
Three Months Ended March 31, 2003 |
Three Months Ended March 31, 2004 |
||||||||||||||||
| |
(dollars in thousands) |
||||||||||||||||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(predecessor and successor combined) |
(successor) |
(successor) |
(successor) |
||||||||||||||||
| Net Income | $ | 42,588 | $ | 9,212 | $ | 14,005 | $ | 23,217 | $ | 36,847 | $ | 16,870 | $ | (485 | ) | ||||||||
| Minority Interest | 725 | 189 | — | 189 | — | — | — | ||||||||||||||||
| Income Taxes | 28,875 | 6,267 | 14,986 | 21,253 | 28,721 | 12,374 | 9,849 | ||||||||||||||||
| Interest (Income) Expenses, Net | (3,413 | ) | (1,364 | ) | 23,898 | 22,534 | 41,468 | 9,948 | 27,372 | ||||||||||||||
| Depreciation and Amortization | 18,056 | 11,722 | 11,424 | 23,146 | 55,605 | 6,389 | 11,406 | ||||||||||||||||
| EBITDA | 86,831 | 26,026 | 64,313 | 90,339 | 162,641 | 45,581 | 48,142 | ||||||||||||||||
| Merger Transaction Expenses(a) | — | 54,708 | 6,183 | 60,891 | — | — | — | ||||||||||||||||
| Other(b) | — | — | — | — | 5,092 | 342 | — | ||||||||||||||||
| Adjusted EBITDA | $ | 86,831 | $ | 80,734 | $ | 70,496 | $ | 151,230 | $ | 167,733 | $ | 45,923 | $ | 48,142 | |||||||||
17
Investing in the Notes involves a high degree of risk. You should carefully consider the following risk factors in addition to the other information contained in this prospectus before deciding to surrender your Outstanding Notes for New Notes pursuant to this exchange offer. The risks described below are not the only ones we face. Other risks, including those that we do not currently consider material or may not currently anticipate, may impair our business.
Risks Related to our Business
Our failure to maintain our distributor relationships could adversely affect our business.
We distribute our products exclusively through independent distributors, and we depend upon them directly for substantially all of our sales. Accordingly, our success depends in significant part upon our ability to attract, retain and motivate a large base of distributors. The loss of a significant number of distributors could materially adversely affect sales of our products and could impair our ability to attract new distributors. Moreover, in our efforts to attract and retain distributors, we compete with other network marketing organizations, including those in the weight management product, dietary and nutritional supplement, and personal care and cosmetic product industries.
Regulatory matters governing our industry could have a significant negative effect on our business.
In both domestic and foreign markets, we are affected by extensive laws, governmental regulations, administrative determinations, court decisions and similar constraints. Such laws, regulations and other constraints may exist at the federal, state or local levels in the United States and at all levels of government in foreign jurisdictions.
Product Regulations.
The formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of our products are subject to extensive regulation by various federal agencies, including the Food and Drug Administration ("FDA"), the Federal Trade Commission (the "FTC"), the Consumer Product Safety Commission and the United States Department of Agriculture and by various agencies of the states, localities and foreign countries in which our products are manufactured, distributed and sold. Our failure or our distributors' failure to comply with those regulations or new regulations could lead to the imposition of significant penalties or claims and could materially adversely affect our business. In addition, the adoption of new regulations or changes in the interpretations of existing regulations may result in significant compliance costs or discontinuation of product sales and may adversely affect the marketing of our products, resulting in significant loss of sales revenues.
Good Manufacturing Practices.
On March 7, 2003, the FDA proposed a new regulation to require current good manufacturing practices in the manufacturing, packing and holding of dietary supplements. We are evaluating this proposal with respect to its potential impact upon the various contract manufacturers that we use to manufacture our products, and cannot assure you of the effect such proposals may have on our contract manufacturers or our business.
Product Claims, Advertising and Distributor Activities.
Our failure to comply with laws and regulations regarding product claims and advertising, including direct claims and advertising by us, as well as claims and advertising and other conduct by distributors for which we may be held responsible, may result in enforcement actions and the imposition of penalties or otherwise materially and adversely affect the distribution and sale of our products. Should
18
distributor activities in our existing markets be found to violate applicable governmental laws or regulations, it could result in governmental or private actions against us in markets where we operate. In response to complaints from local regulators in some of our markets, we imposed a ban in March 2002 on the inappropriate use by distributors of outdoor signage. We cannot assure you as to the effect such ban will have. Given the size of our distributor force, we cannot ensure that all distributors will comply with applicable legal requirements.
Network Marketing System.
Our network marketing system is subject to a number of federal and state regulations administered by the FTC and various state agencies as well as regulations in foreign markets administered by foreign agencies. Regulations applicable to network marketing organizations generally are directed at ensuring that product sales ultimately are made to consumers and that advancement within an organization is based on sales of the organization's products rather than investments in the organization or other non-retail sales related criteria. The regulatory requirements concerning network marketing systems do not include "bright line" rules and are inherently fact-based.
We are also subject to the risk of private party challenges to the legality of our network marketing system. The multi-level marketing programs of other companies have been successfully challenged in the past, and in a current lawsuit, allegations have been made challenging the legality of our network marketing system. An adverse judicial determination with respect to our network marketing system, or in proceedings not involving us directly but which challenge the legality of multi-level marketing systems, could have a material adverse effect on our business. We are subject to the risk that, in one or more markets, our marketing system could be found not to be in compliance with applicable law or regulations. The failure of our network marketing system to comply with such regulations could have a material adverse effect on our business in a particular market or in general.
Transfer Pricing and Similar Regulations.
In many countries, including the United States, we are subject to transfer pricing and other tax regulations designed to ensure that appropriate levels of income are reported as earned by our United States or local entities and are taxed accordingly. In addition, our operations are subject to regulations designed to ensure that appropriate levels of customs duties are assessed on the importation of our products. Herbalife currently is subject to pending or proposed audits that are at various levels of review, assessment or appeal in a number of jurisdictions involving transfer pricing issues, income taxes, value added taxes, withholding taxes and related interest and penalties in material amounts. In some circumstances, additional taxes, interest and penalties have been assessed, and we will be required to litigate to reverse the assessments. Ultimate resolution of these matters may take several years, and the outcome is uncertain.
Taxation Relating to Distributors.
Our distributors are subject to taxation, and in some instances, legislation or governmental agencies impose an obligation on us to collect taxes, such as value added taxes, and to maintain appropriate records. In addition, we are subject to the risk in some jurisdictions of being responsible for social security and similar taxes with respect to our distributors.
Other Regulations.
We are also subject to a variety of other regulations in various foreign markets, including regulations pertaining to employment and severance pay requirements, import/export regulations and antitrust issues. Our failure to comply, or assertions that we failed to comply, with these regulations could have a material adverse effect on our business in a particular market or in general.
19
To the extent we decide to commence or expand operations in additional countries, government regulations in those countries may prevent or delay entry into or expansion of operations in those markets. In addition, the ability to sustain satisfactory levels of sales in our existing markets is dependent in significant part on our ability to introduce additional products into these markets. Government regulations in both our domestic and international markets, however, can delay or prevent the introduction, or require the reformulation or withdrawal, of some of our products.
Adverse publicity associated with our products or our ingredients could adversely affect our business.
Because we are highly dependent upon consumers' perception of the safety and quality of our products and ingredients as well as similar products distributed by other companies, we could be adversely affected if any of our products or any similar products or ingredients distributed by other companies prove to be, or are asserted to be, harmful to consumers. In addition, because of our dependence upon consumer perceptions, any adverse publicity associated with illness or other adverse effects resulting from consumers' use or misuse of our products or any similar products distributed by other companies could have a material adverse impact on our business. Adverse publicity could also negatively affect our ability to attract, motivate and retain distributors.
The high level of competition in our industry could adversely affect our business.
The business of marketing weight management, inner nutrition and Outer Nutrition® products is highly competitive. These market segments include numerous manufacturers, distributors, marketers, retailers and physicians that actively compete for the business of consumers both in the United States and abroad. The market is highly sensitive to the introduction of new products or weight management plans, including various prescription drugs, which may rapidly capture a significant share of the market. While we own the proprietary rights to substantially all of our weight management and inner nutrition products, we cannot be sure that another company will not replicate one of our products. In addition, we anticipate that we will be subject to increasing competition in the future from sellers that utilize electronic commerce.
We are subject to significant competition for the recruitment of distributors from other network marketing organizations, including those that market weight management products, dietary and nutritional supplements, and personal care products as well as other types of products. Our ability to remain competitive depends, in significant part, on our success in recruiting and retaining distributors through an attractive compensation plan and other incentives. We cannot ensure that our programs for recruitment and retention of distributors will be successful.
Risks associated with our foreign operations.
A foreign government may impose trade or foreign exchange restrictions or increased tariffs, which could adversely affect our operations. We are also exposed to risks associated with foreign currency fluctuations. For instance, purchases from suppliers are generally made in U.S. dollars while sales to distributors are generally made in local currencies. Accordingly, strengthening of the U.S. dollar versus a foreign currency could have a negative impact on us. Although we engage in transactions to protect against risks associated with foreign currency fluctuations, we cannot be certain any hedging activity will effectively reduce our exchange rate exposure. Our operations in some markets also may be adversely affected by political, economic and social instability in foreign countries. As we continue to focus on expanding our existing international operations, these and other risks associated with international operations may increase. Approximately 76% of our net sales for the year ended December 31, 2003 were generated outside the United States.
20
A large portion of net sales is concentrated in a small number of countries.
Our earnings in future periods may be susceptible to various risks because of the concentration of net sales in a small number of countries. Of the 58 countries in which we operated as of March 31, 2004, the United States, Japan and Germany accounted for 19.8%, 9.2% and 6.9%, respectively, or 35.9% in the aggregate, of our total net sales. As a result, our performance is primarily dependent upon economic conditions and consumer demand for our products in these three countries.
One of our products constitutes a significant portion of our retail sales.
Our Formula 1 meal replacement product constitutes a significant portion of our retail sales, accounting for approximately 20% of net sales in 2003. If consumer demand for this product decreases significantly or we cease offering this product without a suitable replacement, our operations could be materially adversely affected.
Our ability to grow in the future will be more dependent on increased penetration of existing markets.
We have historically grown principally by entering into new markets. Because we have already succeeded in entering into the most attractive markets for our products and distribution system, an increasingly important part of our strategy for continued growth is to increase the number and range of our products available in existing markets. In addition, our growth will depend upon improved training and other activities that enhance distributor retention in our markets. We cannot assure you that our efforts to increase our market penetration and distributor retention in existing markets will be successful.
In addition, our success has been, and will continue to be, significantly dependent on our ability to manage growth through expansion and enhancement of our worldwide personnel and management, order processing and fulfillment, inventory and shipping systems and other aspects of operations. As we continue to expand our operations, the ability to manage this growth will represent an increasing challenge.
Product liability claims could hurt our business.
Our products consist of herbs, vitamins and minerals and other ingredients that are classified as foods or dietary supplements and are not subject to pre-market regulatory approval in the United States. We generally do not conduct or sponsor clinical studies for our products. As a marketer of dietary and nutritional supplements and other products that are ingested by consumers or applied to their bodies, we have been and may again be subjected to various product liability claims, including that: (i) the products contain contaminants; (ii) the products include inadequate instructions as to their uses; or (iii) the products include inadequate warnings concerning side effects and interactions with other substances. It is possible that widespread product liability claims and the resulting adverse publicity could negatively affect our business. In addition, our product liability insurance may fail to cover future product liability claims thereby requiring us to pay substantial monetary damages and adversely affecting our business (especially given the higher level of self-insurance we have accepted). Finally, we may become required to pay higher premiums and accept higher deductibles in order to secure adequate insurance coverage in the future.
We do not manufacture our own products so we must rely on independent third parties for the manufacture and supply of our products.
All of our products are manufactured by outside companies, except for a small amount of products manufactured in our own manufacturing facility in China. We cannot assure you that these outside manufacturers will continue to reliably supply products to us at the level of quality we require. In the event any of our third-party manufacturers were to become unable or unwilling to continue to provide
21
the products in required volumes and quality levels, we would be required to identify and obtain acceptable replacement manufacturing sources. There is no assurance that we will be able to obtain alternative manufacturing sources on a timely basis. An extended interruption in the supply of products would result in loss of sales. In addition, any actual or perceived degradation of product quality as a result of reliance on third party manufacturers may have an adverse effect on sales or result in increased product returns and buybacks.
Terrorist attacks or acts of war may seriously harm our business.
Terrorist attacks or acts of war may cause damage or disruption to us, our employees, our facilities and our customers, which could impact our revenues, costs and expenses, and financial condition. The terrorist attacks that took place in the United States on September 11, 2001 were unprecedented events that have created many economic and political uncertainties, some of which may materially adversely affect our business, results of operations, and financial condition. The potential for future terrorist attacks, the national and international responses to terrorist attacks, and other acts of war or hostility have created many economic and political uncertainties, which could materially adversely affect our business, results of operations, and financial condition in ways that we currently cannot predict.
A general economic downturn may reduce our revenues.
Worldwide economic conditions may affect demand for our products. Consumer purchases of our products may decline during recessionary periods and also may decline at other times when disposable income is lower.
A few of our shareholders collectively control us and have the power to cause the approval or rejection of all shareholder actions.
Affiliates of Whitney and Golden Gate own approximately 51% and 29%, respectively, of the voting power of our share capital as of March 31, 2004. Accordingly, the Equity Sponsors together currently have the power to cause the approval or rejection of any matter on which the shareholders may vote, including the Transactions. Certain shareholders are party to a shareholders' agreement that determines the composition of the Board of Directors. The Equity Sponsors' designees will constitute the majority of the Board of Directors. This control over corporate actions may delay, deter or prevent transactions that would result in a change of control. In addition, even if all shareholders other than the Equity Sponsors voted together as a group, they would not have the power to adopt any action or to block the adoption of any action favored by the Equity Sponsors if the Equity Sponsors act in concert. Moreover, the Equity Sponsors may have interests that are in addition to, or that vary from, yours.
Risks Related to the Notes
The market value of the New Notes could be materially adversely affected if only a limited number of New Notes are available for trading.
To the extent that a large amount of the Outstanding Notes are not tendered or are tendered and not accepted in the exchange offer, the trading market for the New Notes could be materially adversely affected. Generally, a limited amount, or "float," of a security could result in less demand to purchase such security and, as a result, could result in lower prices for such security. We cannot assure you that a sufficient number of Outstanding Notes will be exchanged for New Notes so that this does not occur.
There are consequences associated with failing to exchange the Outstanding Notes for the New Notes.
If you do not exchange your Outstanding Notes for New Notes in the exchange offer, you will still have the restrictions on transfer provided in the Outstanding Notes and the indenture. In general, the
22
Outstanding Notes may not be offered or sold unless registered or exempt from registration under the Securities Act, or in a transaction not subject to the Securities Act and applicable state securities laws. We do not plan to register the Outstanding Notes under the Securities Act.
Our substantial amount of consolidated debt could adversely affect our consolidated financial condition and prevent us from fulfilling our obligations under the Notes.
In connection with the consummation of the Transactions, we have incurred a substantial amount of debt. At March 31, 2004, our total debt was $510.6 million and our shareholders' equity was $16.4 million.
Our substantial amount of debt may have important consequences for us. For example, it may:
We are a holding company and the Notes are structurally subordinated to the debt of our subsidiaries.
We are a holding company and we conduct substantially all of our operations through our subsidiaries, primarily Herbalife. Our only material assets are our ownership interests in our subsidiaries. Our principal sources of cash are from external financings, dividends and advances from our subsidiaries and investments. The amount of dividends available to us from our subsidiaries depends largely upon each subsidiary's earnings and operating capital requirements. The terms of some of our subsidiaries' borrowing arrangements limit the transfer of funds to us, including Herbalife's senior subordinated notes and senior credit facilities. In addition, the ability of our subsidiaries to make any payments to us will depend on their business and tax considerations and legal restrictions. Our subsidiaries are separate and distinct legal entities and have no obligation, contingent or otherwise, to pay any amounts due pursuant to the Notes or to make any funds available therefor, whether by dividends, loans or other payments.
As a result of our holding company structure, the Notes will effectively rank junior to all existing and future debt, trade payables and other liabilities of our subsidiaries. Our right to receive any assets of any of our subsidiaries upon their liquidation or reorganization, and therefore the right of holders of the Notes to participate in those assets, will be subject to the prior claims of that subsidiary's creditors, including trade creditors. At March 31, 2004, in addition to trade debt and other liabilities, our subsidiaries had approximately $243.2 million of total indebtedness for borrowed money. In addition, the indenture governing the Notes permits, subject to specified limitations, our subsidiaries to incur additional debt.
23
Your right to receive payment on the Notes is junior to our secured obligations under our guarantee of our subsidiaries' senior credit facilities.
The Notes will be general unsecured obligations, junior in right of payment to all of our obligations under a guaranty of our subsidiaries' senior credit facilities. The Notes will not be secured by any of our assets, and as such will be effectively subordinated to any secured debt that we have now, including our guaranty of the borrowings under our subsidiaries' senior credit facilities, or may incur in the future to the extent of the value of the assets securing that debt.
In the event that we are declared bankrupt, become insolvent or are liquidated or reorganized, any debt that ranks ahead of the Notes will be entitled to be paid in full from our assets before any payment may be made with respect to the Notes. In any of the foregoing events, we cannot assure you that we would have sufficient assets to pay amounts due on the Notes. As a result, holders of the Notes may receive less, proportionally, than the holders of debt senior to the Notes. The subordination provisions of the indenture governing the Notes also provide that we can make no payment to you during the continuance of payment defaults on our senior debt, and payments to you may be suspended for a period of up to 179 days if a nonpayment default exists under our subsidiaries' senior credit facilities.
At March 31, 2004, the Outstanding Notes ranked junior to approximately $75.4 million of indebtedness under the senior credit facilities, all of which is secured.
To make payments on our debt, we will require a significant amount of cash; our ability to generate cash depends on many factors beyond our control.
Our ability to make payments on our debt, including the Notes, will depend on our ability to generate cash in the future. This, to some extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control.
We cannot assure you that our subsidiaries' businesses will generate sufficient cash flow from operations or that future borrowings will be available to us in an amount sufficient to enable us to pay interest and principal on our debt or to fund our other liquidity needs. We may need to refinance all or a portion of our debt on or before maturity. We cannot assure you that we will be able to refinance any of our debt on commercially reasonable terms or at all.
We may not have access to the cash flow and other assets of our subsidiaries that may be needed to make payment on the Notes.
Our operations are conducted through our subsidiaries and our ability to make payment on the Notes is dependent on the earnings and the distribution of funds from our subsidiaries. However, none of our subsidiaries is obligated to make funds available to us for payment on the Notes. In addition, the terms of the agreements governing Herbalife's senior credit facilities and senior subordinated Notes significantly restrict our subsidiaries from paying dividends and otherwise transferring assets to us. Furthermore, Herbalife will be permitted under the terms of its senior credit facilities, senior subordinated notes and other indebtedness to incur additional indebtedness that may severely restrict or prohibit the making of distributions, the payment of dividends or the making of loans by our subsidiaries to us.
We cannot assure you that the agreements governing the current and future indebtedness of our subsidiaries will permit our subsidiaries to provide us with sufficient dividends, distributions or loans to fund scheduled interest and principal payments on the Notes when due. See "Description of Other Indebtedness."
24
The ability of some of our foreign subsidiaries to distribute cash may be restricted by local law.
Local laws governing some of our foreign subsidiaries may restrict the ability of those foreign subsidiaries to pay dividends and make distributions, loans or advances to us in some circumstances. For example, some foreign subsidiaries could be subject to restrictions on dividends or repatriations of earnings under applicable local law, monetary transfer restrictions and foreign currency exchange regulations in the jurisdictions in which these foreign subsidiaries operate. In any of the foregoing events, we cannot assure you that we would have sufficient assets to pay amounts due on the Notes.
The covenants in the Notes will limit, and the covenants in our subsidiaries' senior credit facilities limit, our and their discretion with respect to certain business matters.
The Notes will contain and our subsidiaries' senior credit facilities contain numerous financial and operating covenants that will restrict our and their ability to, among other things:
In addition, our subsidiaries' senior credit facilities require us to meet certain financial ratios and financial conditions, including minimum interest charge and fixed charge ratios and a maximum leverage ratio. Our and their ability to comply with these covenants may be affected by events beyond our control, including prevailing economic, financial and industry conditions. Failure to comply with these covenants could result in a default under the Notes and/or the senior credit facilities, causing all amounts thereunder to become due and payable.
Issuance of the Notes and the guarantees issued under certain circumstances by our domestic subsidiaries may be subject to fraudulent conveyance laws.
Under applicable provisions of the U.S. Bankruptcy Code or comparable provisions of state fraudulent transfer or conveyance laws, if at the time the issuer of the Notes incurred the debt evidenced by the Notes, or a domestic subsidiary guarantor incurred the debt evidenced by its guarantee, as the case may be, it either:
then, in each case, a court of competent jurisdiction could (i) avoid (i.e., cancel) in whole or in part, the Notes or the guarantee and direct the repayment of any amounts paid thereunder, (ii) subordinate
25
the Notes or the guarantee of such subsidiary guarantor, as the case may be, to our obligations to other existing and future creditors or (iii) take other actions detrimental to the noteholders.
A court would likely find that neither we nor any subsidiary guarantor received reasonably equivalent value or fair consideration for incurring our respective obligations under the Notes and guarantees unless we or the subsidiary benefited directly or indirectly from the Notes' issuance, including the application of the proceeds thereof. In other instances, courts have found that an issuer did not receive reasonably equivalent value or fair consideration if the proceeds of the issuance were paid to the issuer's shareholders, although we cannot predict how a court would rule in this case.
The test for determining solvency for purposes of the foregoing will vary depending on the law of the jurisdiction being applied. In general, a court would consider an entity insolvent either if the sum of its existing debts exceeds the fair value of all its property, or its assets' present fair saleable value is less than the amount required to pay the probable liability on its existing debts as they become due. For this analysis, "debts" includes contingent and unliquidated debts.
In the event that any of our subsidiaries are required to guarantee our obligations under the Notes, such guarantee will be limited in a manner intended to avoid it being deemed a fraudulent conveyance under applicable law.
We may not have the ability to raise the funds necessary to finance a change of control offer required by the indenture governing the Notes.
Upon the occurrence of specific change of control events, we may be required to purchase all Notes at 101% of their principal amount. It is possible that we will not have sufficient funds at the time of a change of control to purchase the Notes or that restrictions in our or our subsidiaries' other debt agreements will not allow the purchases. If we are unable to purchase the Notes upon a change of control, we would be in default under the indenture governing the Notes, which could cause acceleration of our other debt. In addition, some important corporate events, such as leveraged recapitalizations that would increase the level of our debt, would not necessarily constitute a "Change of Control" under the indenture governing the Notes.
You cannot be sure that an active trading market will develop for the Notes and you may have to hold the Notes indefinitely.
The Notes are a new issue of securities for which there currently is no trading market. As a result, we cannot provide any assurances that a market will develop for the Notes or that you will be able to sell your Notes. If any of the Notes are traded after their initial issuance, they may trade at a discount from their initial offering price. Future trading prices of the Notes will depend on many factors, including prevailing interest rates, the market for similar securities, general economic conditions and our financial condition, performance and prospects. Historically, the market for non-investment grade debt has been subject to disruptions that have caused substantial fluctuations in the prices of the securities. Accordingly, you may be required to bear the financial risk of an investment in the Notes for an indefinite period of time.
We do not intend to apply for listing or quotation of the Notes. The Notes have been designated, however, for trading in The PORTAL® Market. We have been informed by the initial purchaser of the Outstanding Notes that it intends to make a market in the New Notes after this exchange offer. The initial purchaser is not obligated to do so, and it may cease its market-making at any time without notice. In addition, this market-making activity will be subject to the limitations imposed by the Securities Act and the Exchange Act and may be limited during the effectiveness of a registration statement relating to the Notes.
26
There is uncertainty as to your ability to enforce certain foreign civil liabilities in the Cayman Islands.
We are incorporated as an exempted company with limited liability under the laws of the Cayman Islands. A material portion of our assets are located outside of the United States. As a result, it may be difficult for persons purchasing New Notes (or persons who purchased Outstanding Notes and exchange them for New Notes) to enforce judgments against us or judgments obtained in U.S. courts predicated upon the civil liability provisions of the federal securities laws of the United States or any state of the United States.
We have been advised by our Cayman Islands counsel, Maples and Calder, that although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will—based on the principle that a judgment by a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given—recognize and enforce a foreign judgment of a court of competent jurisdiction if such judgment is final, for a liquidated sum, not in respect of taxes or a fine or penalty, is not inconsistent with a Cayman Islands judgment in respect of the same matters, and was not obtained in a manner, and is not of a kind, the enforcement of which is contrary to the public policy of the Cayman Islands. There is doubt, however, as to whether the Grand Court of the Cayman Islands will (i) recognize or enforce judgments of U.S. courts predicated upon the civil liability provisions of the securities laws of the United States or any state of the United States, or (ii) in original actions brought in the Cayman Islands, impose liabilities predicated upon the civil liability provisions of the securities laws of the United States or any state of the United States, on the grounds that such provisions are penal in nature.
The Grand Court of the Cayman Islands may stay proceedings if concurrent proceedings are being brought elsewhere.
27
THE RECAPITALIZATION OF HOLDINGS AND RELATED TRANSACTIONS
The proceeds of the offering of the Outstanding Notes, together with available cash, were used to consummate (i) the redemption of all of Holdings' outstanding 12% Series A Cumulative Convertible Preferred Shares, which we refer to herein as the "Preferred Shares," including Preferred Shares issued in connection with the exercise of all outstanding warrants to purchase the Preferred Shares, which we refer to herein as the "Warrants;" (ii) the purchase of all of Holdings' outstanding Senior Notes; (iii) the reduction of outstanding amounts under the term loan under Herbalife's senior credit facilities; and (iv) the payment of related fees and expenses (collectively, the "Transactions").
Immediately following the Transactions, Holdings no longer had any Preferred Shares or any Warrants to purchase the Preferred Shares outstanding, and Holdings' material obligations principally consisted of the obligations incurred under the Outstanding Notes and its guarantee of Herbalife's senior credit facilities.
Prior to consummating the Transactions, Herbalife amended and restated the credit agreement governing its senior credit facilities to facilitate the Transactions described above. See "Description of Other Indebtedness—Herbalife's Senior Credit Facilities."
28
Exchange Terms
We sold the Outstanding Notes on March 8, 2004 to the initial purchaser pursuant to a purchase agreement. The initial purchaser subsequently sold the Outstanding Notes to:
As a condition to the initial sale of the Outstanding Notes, Holdings, WH Capital Corp. and the initial purchaser entered into a registration rights agreement. Pursuant to the registration rights agreement, we agreed to:
We agreed to issue and exchange the New Notes for all Outstanding Notes properly surrendered and not withdrawn before the expiration of the exchange offer. A copy of the registration rights agreement has been filed as an exhibit to the registration statement which includes this prospectus. The registration statement is intended to satisfy some of our obligations under the registration rights agreement and the purchase agreement.
Outstanding Notes in an aggregate principal amount of $275,000,000 are currently issued and outstanding. The maximum aggregate principal amount of New Notes that will be issued in exchange for Outstanding Notes is $275,000,000. The terms of the New Notes and the Outstanding Notes are substantially the same in all material respects, except that the New Notes will be freely transferable by the holders except as provided in this prospectus. See "Description of Notes."
The New Notes will bear interest at a rate of 91/2% per year, payable semiannually on April 1 and October 1 of each year, beginning on October 1, 2004. Holders of New Notes will receive interest from the date of the original issuance of the Outstanding Notes or from the date of the last payment of interest on the Outstanding Notes, whichever is later. Holders of New Notes will not receive any interest on Outstanding Notes tendered and accepted for exchange. In order to exchange your Outstanding Notes for transferable New Notes in the exchange offer, you will be required to make the following representations, which are included in the letter of transmittal:
29
Upon the terms and subject to the conditions set forth in this prospectus and in the letter of transmittal, we will accept for exchange any Outstanding Notes properly tendered and not validly withdrawn in the exchange offer, and the exchange agent will deliver the New Notes promptly after the Expiration Date of the exchange offer.
If you tender your Outstanding Notes, you will not be required to pay brokerage commissions or fees or, subject to the instructions in the letter of transmittal, transfer taxes with respect to the exchange of the Outstanding Notes in connection with the exchange offer. We will pay all charges, expenses and transfer taxes in connection with the exchange offer, other than the taxes described below under "—Transfer Taxes."
WE MAKE NO RECOMMENDATION TO YOU AS TO WHETHER YOU SHOULD TENDER OR REFRAIN FROM TENDERING ALL OR ANY PORTION OF YOUR OUTSTANDING NOTES INTO THIS EXCHANGE OFFER. IN ADDITION, NO ONE HAS BEEN AUTHORIZED TO MAKE THIS RECOMMENDATION. YOU MUST MAKE YOUR OWN DECISION WHETHER TO TENDER INTO THIS EXCHANGE OFFER AND, IF SO, THE AGGREGATE AMOUNT OF OUTSTANDING NOTES TO TENDER AFTER READING THIS PROSPECTUS AND THE LETTER OF TRANSMITTAL AND CONSULTING WITH YOUR ADVISORS, IF ANY, BASED ON YOUR FINANCIAL POSITION AND REQUIREMENTS.
Expiration Date; Extensions; Termination; Amendments
The exchange offer expires at 5:00 p.m., New York City time, on Wednesday, July 9, 2004, unless we extend the exchange offer, in which case the Expiration Date will be the latest date and time to which we extend the exchange offer.
In order to extend the exchange offer, we will:
We expressly reserve the right, so long as applicable law allows:
Any waiver or amendment to the exchange offer will apply to all Outstanding Notes tendered, regardless of when or in what order the Outstanding Notes were tendered. If the exchange offer is amended in a manner that we think constitutes a material change, or if we waive a material condition of the exchange offer, we will promptly disclose the amendment or waiver by means of a prospectus supplement that will be distributed to the registered holders of the Outstanding Notes, and we will extend the exchange offer to the extent required by Rule 14e-1 under the Exchange Act.
30
We will promptly follow any delay in acceptance, termination, extension or amendment by oral or written notice of the event to the exchange agent, followed promptly by oral or written notice to the registered holders. Should we choose to delay, extend, amend or terminate the exchange offer, we will have no obligation to publish, advertise or otherwise communicate this announcement, other than by making a timely release to an appropriate news agency.
In the event we terminate the exchange offer, all Outstanding Notes previously tendered and not accepted for payment will be returned promptly to the tendering holders.
In the event that the exchange offer is withdrawn or otherwise not completed, New Notes will not be given to holders of Outstanding Notes who have validly tendered their Outstanding Notes.
Resale Of New Notes
Based on interpretations of the SEC staff set forth in no action letters issued to third parties, we believe that New Notes issued under the exchange offer in exchange for Outstanding Notes may be offered for resale, resold and otherwise transferred by you without compliance with the registration and prospectus delivery requirements of the Securities Act, if:
However, we have not asked the SEC to consider this particular exchange offer in the context of a no-action letter. Therefore, you cannot be sure that the SEC will treat it in the same way it has treated other exchange offers in the past.
If you tender Outstanding Notes in the exchange offer with the intention of participating in any manner in a distribution of the New Notes:
Only broker-dealers that acquired the Outstanding Notes as a result of market-making activities or other trading activities may participate in the exchange offer. Each broker-dealer that receives New Notes for its own account in exchange for Outstanding Notes, where such Outstanding Notes were acquired by such broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of the New Notes. Please read the section captioned "Plan of Distribution" on page 160 for more details regarding the transfer of New Notes.
Acceptance Of Outstanding Notes For Exchange
We will accept for exchange Outstanding Notes validly tendered pursuant to the exchange offer, or defectively tendered, if such defect has been waived by us, after the later of: (1) the Expiration Date of the exchange offer and (2) the satisfaction or waiver of the conditions specified below under "—Conditions Of The Exchange Offer." We will not accept Outstanding Notes for exchange
31
subsequent to the Expiration Date of the exchange offer. Tenders of Outstanding Notes will be accepted only in minimum denominations equal to $100,000 or integral multiples of $1,000 in excess thereof.
We expressly reserve the right, in our sole discretion, to:
In all cases, New Notes will be issued only after timely receipt by the exchange agent of certificates representing Outstanding Notes, or confirmation of book-entry transfer, a properly completed and duly executed letter of transmittal, or a manually signed facsimile thereof, and any other required documents. For purposes of the exchange offer, we will be deemed to have accepted for exchange validly tendered Outstanding Notes, or defectively tendered Outstanding Notes with respect to which we have waived such defect, if, as and when we give oral, confirmed in writing, or written notice to the exchange agent. Promptly after the Expiration Date, we will deposit the New Notes with the exchange agent, who will act as agent for the tendering holders for the purpose of receiving the New Notes and transmitting them to the holders. The exchange agent will deliver the New Notes to holders of Outstanding Notes accepted for exchange after the exchange agent receives the New Notes.
If, for any reason, we delay acceptance for exchange of validly tendered Outstanding Notes or we are unable to accept for exchange validly tendered Outstanding Notes, then the exchange agent may, nevertheless, on our behalf, retain tendered Outstanding Notes, without prejudice to our rights described under "—Expiration Date; Extensions; Termination; Amendments" beginning on page 29, "—Conditions Of The Exchange Offer" beginning on page 35 and "—Withdrawal Of Tenders" beginning on page 34, subject to Rule 14e-1 under the Exchange Act, which requires that an offeror pay the consideration offered or return the securities deposited by or on behalf of the holders thereof promptly after the termination or withdrawal of a tender offer.
If any tendered Outstanding Notes are not accepted for exchange for any reason, or if certificates are submitted evidencing more Outstanding Notes than those that are tendered, certificates evidencing Outstanding Notes that are not exchanged will be returned, without expense, to the tendering holder, or, in the case of Outstanding Notes tendered by book-entry transfer into the exchange agent's account at a book-entry transfer facility under the procedure set forth under "—Procedures for Tendering Outstanding Notes—Book-Entry Transfer" on page 33, such Outstanding Notes will be credited to the account maintained at such book-entry transfer facility from which such Outstanding Notes were delivered, unless otherwise requested by such holder under "Special Delivery Instructions" in the letter of transmittal, promptly following the exchange date or the termination of the exchange offer.
Tendering holders of Outstanding Notes exchanged in the exchange offer will not be obligated to pay brokerage commissions or transfer taxes with respect to the exchange of their Outstanding Notes other than as described in "—Transfer Taxes" on page 36 or in Instruction 7 to the letter of transmittal. We will pay all other charges and expenses in connection with the exchange offer.
Procedures For Tendering Outstanding Notes
Any beneficial owner whose Outstanding Notes are registered in the name of a broker, dealer, commercial bank, trust company or other nominee or held through a book-entry transfer facility and
32
who wishes to tender Outstanding Notes should contact such registered holder promptly and instruct such registered holder to tender Outstanding Notes on such beneficial owner's behalf.
Tender of Outstanding Notes Held Through Depository Trust. The exchange agent and Depository Trust have confirmed that the exchange offer is eligible for the Depository Trust automated tender offer program. Accordingly, Depository Trust participants may electronically transmit their acceptance of the exchange offer by causing Depository Trust to transfer Outstanding Notes to the exchange agent in accordance with Depository Trust's automated tender offer program procedures for transfer. Depository Trust will then send an agent's message to the exchange agent.
The term "agent's message" means a message transmitted by Depository Trust, received by the exchange agent and forming part of the book-entry confirmation, which states that Depository Trust has received an express acknowledgment from the participant in Depository Trust tendering Outstanding Notes that are the subject of that book-entry confirmation that the participant has received and agrees to be bound by the terms of the letter of transmittal, and that we may enforce such agreement against such participant. In the case of an agent's message relating to guaranteed delivery, the term means a message transmitted by Depository Trust and received by the exchange agent which states that Depository Trust has received an express acknowledgment from the participant in Depository Trust tendering Outstanding Notes that they have received and agree to be bound by the notice of guaranteed delivery.
Tender of Outstanding Notes Held in Certificated Form. For a holder to validly tender Outstanding Notes held in certificated form:
LETTERS OF TRANSMITTAL AND OUTSTANDING NOTES SHOULD BE SENT ONLY TO THE EXCHANGE AGENT, AND NOT TO US OR TO ANY BOOK-ENTRY TRANSFER FACILITY.
THE METHOD OF DELIVERY OF OUTSTANDING NOTES, LETTERS OF TRANSMITTAL AND ALL OTHER REQUIRED DOCUMENTS TO THE EXCHANGE AGENT IS AT THE ELECTION AND RISK OF THE HOLDER TENDERING OUTSTANDING NOTES. DELIVERY OF SUCH DOCUMENTS WILL BE DEEMED MADE ONLY WHEN ACTUALLY RECEIVED BY THE EXCHANGE AGENT. IF SUCH DELIVERY IS BY MAIL, WE SUGGEST THAT THE HOLDER USE PROPERLY INSURED, REGISTERED MAIL WITH RETURN RECEIPT REQUESTED, AND THAT THE MAILING BE MADE SUFFICIENTLY IN ADVANCE OF THE EXPIRATION DATE OF THE EXCHANGE OFFER TO PERMIT DELIVERY TO THE EXCHANGE AGENT PRIOR TO SUCH DATE. NO ALTERNATIVE, CONDITIONAL OR CONTINGENT TENDERS OF OUTSTANDING NOTES WILL BE ACCEPTED.
Signature Guarantee. Signatures on the letter of transmittal must be guaranteed by an eligible institution unless:
33
An eligible institution is a firm that is a participant in the Security Transfer Agents Medallion program or the Stock Exchange Medallion program, which is generally a member of a registered national securities exchange, a member of the National Association of Securities Dealers, Inc., or a commercial bank or trust company having an office in the United States.
Book-Entry Transfer. The exchange agent will seek to establish a new account or utilize an existing account with respect to the Outstanding Notes at Depository Trust promptly after the date of this prospectus. Any financial institution that is a participant in the book-entry transfer facility system and whose name appears on a security position listing it as the owner of the Outstanding Notes must make book-entry delivery of Outstanding Notes by causing the book-entry transfer facility to transfer such Outstanding Notes into the exchange agent's account. HOWEVER, ALTHOUGH DELIVERY OF OUTSTANDING NOTES MAY BE EFFECTED THROUGH BOOK-ENTRY TRANSFER INTO THE EXCHANGE AGENT'S ACCOUNT AT A BOOK-ENTRY TRANSFER FACILITY, A PROPERLY COMPLETED AND VALIDLY EXECUTED LETTER OF TRANSMITTAL, OR A MANUALLY SIGNED FACSIMILE THEREOF, MUST BE RECEIVED BY THE EXCHANGE AGENT AT ONE OF ITS ADDRESSES SET FORTH IN THIS PROSPECTUS ON OR PRIOR TO THE EXPIRATION DATE OF THE EXCHANGE OFFER, OR ELSE THE GUARANTEED DELIVERY PROCEDURES DESCRIBED BELOW MUST BE COMPLIED WITH. The confirmation of a book-entry transfer of Outstanding Notes into the exchange agent's account at a book-entry transfer facility is referred to in this prospectus as a "book-entry confirmation." Delivery of documents to the book-entry transfer facility in accordance with that book-entry transfer facility's procedures does not constitute delivery to the exchange agent.
Guaranteed Delivery. If you wish to tender your Outstanding Notes and:
34
Other Matters. New Notes will be issued in exchange for Outstanding Notes accepted for exchange only after timely receipt by the exchange agent of:
We will determine, in our sole discretion, all questions as to the form of all documents, validity, eligibility, including time of receipt, and acceptance of all tenders of Outstanding Notes. Our determination will be final and binding on all parties. ALTERNATIVE, CONDITIONAL OR CONTINGENT TENDERS OF OUTSTANDING NOTES WILL NOT BE CONSIDERED VALID. We reserve the absolute right to reject any or all tenders of Outstanding Notes that are not in proper form or the acceptance of which, in our opinion, would be unlawful. We also reserve the right to waive any defects, irregularities or conditions of tender as to particular Outstanding Notes.
Our interpretation of the terms and conditions of the exchange offer, including the instructions in the letter of transmittal, will be final and binding.
Any defect or irregularity in connection with tenders of Outstanding Notes must be cured within the time we determine, unless waived by us. We will not consider the tender of Outstanding Notes to have been validly made until all defects and irregularities have been waived by us or cured. Neither we, the exchange agent, or any other person will be under any duty to give notice of any defects or irregularities in tenders of Outstanding Notes, or will incur any liability to holders for failure to give any such notice.
Withdrawal Of Tenders
Except as otherwise provided in this prospectus, you may withdraw your tender of Outstanding Notes at any time prior to the Expiration Date.
For a withdrawal to be effective:
35
Any notice of withdrawal must:
If certificates for Outstanding Notes have been delivered or otherwise identified to the exchange agent, then, prior to the release of such certificates, the withdrawing holder must also submit the serial numbers of the particular certificates to be withdrawn and a signed notice of withdrawal with signatures guaranteed by an eligible institution unless such holder is an eligible institution. If Outstanding Notes have been tendered pursuant to the procedure for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at Depository Trust to be credited with the withdrawn Outstanding Notes and otherwise comply with the procedures of such Depository Trust.
If Outstanding Notes have been tendered pursuant to the procedure for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at Depository Trust to be credited with the withdrawn Outstanding Notes and otherwise comply with the procedures of Depository Trust.
We will determine all questions as to validity, form, eligibility and time of receipt of any withdrawal notices. Our determination will be final and binding on all parties. We will deem any Outstanding Notes so withdrawn not to have been validly tendered for exchange for purposes of the exchange offer.
Any Outstanding Notes that have been tendered for exchange but that are not exchanged for any reason will be returned to their holder without cost to the holder or, in the case of Outstanding Notes tendered by book-entry transfer into the exchange agent's account at Depository Trust according to the procedures described above, such Outstanding Notes will be credited to an account maintained with Depository Trust for the Outstanding Notes. This return or crediting will take place as soon as practicable after withdrawal, rejection of tender or termination of the exchange offer. You may retender properly withdrawn Outstanding Notes by following one of the procedures described under "—Procedures for Tendering Outstanding Notes" beginning on page 32 at any time on or prior to the Expiration Date.
Conditions Of The Exchange Offer
We may terminate, waive any conditions to or amend the exchange offer or, subject to Rule 14e-1 under the Exchange Act which requires that an offeror pay the consideration offered or return the securities deposited by or on behalf of the holders thereof promptly after the termination or withdrawal of the exchange offer, postpone the acceptance for exchange of Outstanding Notes so tendered if, on or prior to the Expiration Date of the exchange offer, we determine that the exchange offer would violate applicable law or any applicable interpretation of the staff of the SEC. We reserve the right to waive any defects, irregularities or conditions of surrender as to particular Outstanding Notes.
Transfer Taxes
We will pay all transfer taxes applicable to the transfer and exchange of Outstanding Notes pursuant to the exchange offer. If, however:
36
the amount of any such transfer taxes, whether imposed on the record holder or any other person, will be payable by the tendering holder prior to the issuance of the New Notes.
Consequences Of Failing To Exchange
If you do not exchange your Outstanding Notes for New Notes in the exchange offer, you will remain subject to the restrictions on transfer of the Outstanding Notes:
In general, you may not offer or sell the Outstanding Notes unless they are registered under the Securities Act, or if the offer or sale is exempt from registration under the Securities Act and applicable state securities laws. Except as required by the registration rights agreement, we do not intend to register resales of the Outstanding Notes under the Securities Act.
Accounting Treatment
The New Notes will be recorded at the same carrying value as the Outstanding Notes, as reflected in our accounting records on the date of the exchange. Accordingly, we will not recognize any gain or loss for accounting purposes upon the consummation of the exchange offer. We will amortize the expenses of the exchange offer over the term of the New Notes.
Exchange Agent
The Bank of New York has been appointed as exchange agent for the exchange offer. You should direct questions and requests for assistance, requests for additional copies of this prospectus, the letter of transmittal or any other documents to the exchange agent. You should send certificates for Outstanding Notes, letters of transmittal and any other required documents to the exchange agent addressed as follows:
37
The Bank of New York
| |
By Facsimile: |
|
|---|---|---|
| The Bank of New York Corporate Trust Operations Reorganization Unit 101 Barclay Street, 7 East New York, N.Y. 10286 Attn: Ms. Carolle Montreuil |
(for eligible institutions only) (212) 298-1915 Confirm by telephone: (212) 815-5920 |
|
38
We will not receive any proceeds from the exchange offer. The proceeds received by us from the sale of the Outstanding Notes were approximately $275.0 million. We used the proceeds from the offering of the Outstanding Notes, together with available cash, to consummate the Transactions.
The following table summarizes the sources and uses of funds for the Transactions as of the March 8, 2004 closing date for the Transactions.
| |
Amount |
|||
|---|---|---|---|---|
| |
(dollars in millions) |
|||
| Sources of funds | ||||
| Outstanding Notes | $ | 275.0 | ||
| Available cash(1) | 48.8 | |||
| Total sources | $ | 323.8 | ||
| Uses of funds | ||||
| Redeem Holdings' Preferred Shares and pay accrued dividends(2) | $ | 221.6 | ||
| Purchase Holdings' Senior Notes and pay accrued interest(3) | 52.1 | |||
| Reduce Herbalife term loan(4) | 40.0 | |||
| Pay fees and expenses | 10.1 | |||
| Total uses | $ | 323.8 | ||
39
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2004. You should read this table in conjunction with "Use of Proceeds," "Unaudited Pro Forma Condensed Consolidated Financial Statements," "Selected Consolidated Historical Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Certain Relationships and Related Transactions—Redemption of Preferred Shares," "Certain Relationships and Related Transactions—Purchase of Senior Notes" and our consolidated financial statements, and related Notes included elsewhere in this prospectus.
| |
At March 31, 2004 |
||||
|---|---|---|---|---|---|
| |
Actual |
||||
| |
(dollars in millions) |
||||
| Cash and cash equivalents | $ | 123.0 | |||
Debt: |
|||||
| Revolving Credit Facility | — | ||||
| Term Loan Borrowings | 75.4 | ||||
| Capitalized Leases and Other Debt | 9.6 | ||||
| Senior Subordinated Notes, net(1) | 158.2 | ||||
| Outstanding Notes(2) | 267.5 | ||||
| Total Debt | 510.7 | ||||
| Total Equity | 16.8 | ||||
| Total Capitalization | $ | 527.5 | |||
40
SELECTED CONSOLIDATED HISTORICAL FINANCIAL DATA
The following table sets forth certain of our historical financial data. We have derived the selected historical consolidated financial data as of December 31, 2002 and 2003 and for the year ended December 31, 2001, the seven month period ended July 31, 2002, the five month period ended December 31, 2002 and the year ended December 31, 2003 from our audited financial statements and the related notes included elsewhere in this prospectus. The selected historical consolidated financial data as of December 31, 1999, 2000 and 2001 and for the years ended December 31, 1999 and 2000 have been derived from our audited financial statements for such years, which are not included in this prospectus. We have derived the selected historical consolidated financial data for the three months ended March 31, 2003 and as of and for the three months ended March 31, 2004 from our unaudited consolidated financial statements and the related notes included elsewhere in this prospectus. The selected consolidated historical financial data set forth below are not necessarily indicative of the results of future operations and should be read in conjunction with the discussion under the heading "Management's Discussion and Analysis of Financial Condition and Results of Operations," and the historical consolidated financial statements and accompanying notes included elsewhere in this prospectus.
We present EBITDA and adjusted EBITDA because management believes it provides useful information regarding our ability to service and/or incur debt and that it provides a more comparable measure of our profitability. However, such a measure is not in accordance with GAAP. You should not consider EBITDA in isolation from or as a substitute for net income, cash flows from operating activities and other consolidated income or cash flow statement data prepared in accordance with accounting principles generally accepted in the United States or as a measure of profitability or liquidity. Adjusted EBITDA is calculated by adding back to EBITDA buy-out transaction expenses and other non-recurring expenses relating to the acquisition. Adjusted EBITDA, as presented, may not be comparable to similarly titled measures reported by other companies.
| |
Year ended December 31, |
January 1 to July 31, |
August 1 to December 31, |
Year ended December 31, |
Three months ended March 31, |
Three months ended March 31, |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
1999 |
2000 |
2001 |
2002 |
2002 |
2003 |
2003 |
2004 |
||||||||||||||||||
| |
(predecessor) |
(predecessor) |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
(successor) |
(successor) |
||||||||||||||||||
| |
(dollars in thousands) |
|||||||||||||||||||||||||
| Operations: | ||||||||||||||||||||||||||
| Net sales(1) | $ | 1,098,885 | $ | 1,085,484 | $ | 1,020,130 | $ | 644,188 | $ | 449,524 | $ | 1,159,433 | $ | 280,039 | $ | 324,053 | ||||||||||
| Cost of sales | 264,909 | 268,992 | 241,522 | 140,553 | 95,001 | 235,785 | 56,960 | 63,618 | ||||||||||||||||||
| Gross profit | 833,976 | 816,492 | 778,608 | 503,635 | 354,523 | 923,648 | 223,079 | 260,435 | ||||||||||||||||||
| Royalty overrides | 397,143 | 382,322 | 355,225 | 227,233 | 159,915 | 415,351 | 99,511 | 115,857 | ||||||||||||||||||
| Marketing, distribution and administrative expenses(2) | 344,260 | 363,731 | 354,608 | 207,390 | 135,536 | 401,261 | 84,376 | 107,842 | ||||||||||||||||||
| Merger transaction expenses(3) | — | 9,498 | — | 54,708 | 6,183 | — | — | — | ||||||||||||||||||
| Operating income(2) | 92,573 | 60,941 | 68,775 | 14,304 | 52,889 | 107,036 | 39,192 | 36,736 | ||||||||||||||||||
| Interest income (expense), net | 1,750 | 2,354 | 3,413 | 1,364 | (23,898 | ) | (41,468 | ) | (9,947 | ) | (27,372 | ) | ||||||||||||||
| Income before income taxes and minority interest | 94,323 | 63,295 | 72,188 | 15,668 | 28,991 | 65,568 | 29,245 | 9,364 | ||||||||||||||||||
| Income taxes | 36,314 | 25,318 | 28,875 | 6,267 | 14,986 | 28,721 | 12,375 | 9,849 | ||||||||||||||||||
| Income (loss) before minority interest | 58,009 | 37,977 | 43,313 | 9,401 | 14,005 | 36,847 | 16,870 | (485 | ) | |||||||||||||||||
| Minority interest | 1,086 | 1,058 | 725 | 189 | — | — | — | — | ||||||||||||||||||
| Net income (loss) | $ | 56,923 | $ | 36,919 | $ | 42,588 | $ | 9,212 | $ | 14,005 | $ | 36,847 | $ | 16,870 | $ | (485 | ) | |||||||||
| Other Financial Data: | ||||||||||||||||||||||||||
| EBITDA(4) | 106,574 | 76,643 | 86,831 | 26,026 | 64,313 | 162,641 | 45,581 | 48,142 | ||||||||||||||||||
| Adjusted EBITDA(4) | $ | 106,574 | $ | 86,132 | $ | 86,831 | $ | 80,734 | $ | 70,496 | $ | 167,733 | $ | 45,923 | $ | 48,142 | ||||||||||
| Net cash provided by (used in): | ||||||||||||||||||||||||||
| Operating activities | 95,414 | 46,141 | 95,465 | 37,901 | 28,039 | 94,648 | (3,506 | ) | 25,720 | |||||||||||||||||
| Investing activities | (43,517 | ) | (49,968 | ) | (16,366 | ) | 18,995 | (456,046 | ) | 2,854 | 11,082 | (1,549 | ) | |||||||||||||
| Financing activities | (16,041 | ) | (14,079 | ) | (3,456 | ) | (35,292 | ) | 491,519 | (18,831 | ) | (1,770 | ) | (50,198 | ) | |||||||||||
| Depreciation and amortization | 14,001 | 15,693 | 18,056 | 11,722 | 11,424 | 55,605 | 6,389 | 11,406 | ||||||||||||||||||
| Capital expenditures(5) | 32,607 | 25,383 | 14,751 | 6,799 | 3,599 | 20,435 | 2,720 | 5,422 | ||||||||||||||||||
| Ratio of earnings to fixed charges(6) |
11.5 | 8.1 | 8.6 | 3.6 | 2.0 | 2.2 | 3.2 | 1.4 | ||||||||||||||||||
41
| |
As of December 31, |
As of December 31, |
As of December 31, |
As of March 31, |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
|||||||||||
| |
(predecessor) |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
(successor) |
|||||||||||
| |
(dollars in thousands) |
||||||||||||||||
| Balance Sheet Data (at December 31): | |||||||||||||||||
| Cash and cash equivalents(7) | $ | 139,443 | $ | 140,250 | $ | 201,181 | $ | 76,024 | $ | 156,380 | 123,002 | ||||||
| Receivables, net | 30,326 | 24,600 | 27,609 | 29,026 | 31,977 | 33,775 | |||||||||||
| Inventories | 101,557 | 99,332 | 72,208 | 56,868 | 59,397 | 64,134 | |||||||||||
| Total working capital | 133,137 | 145,211 | 177,813 | 7,186 | 1,521 | 18,789 | |||||||||||
| Total assets | 415,819 | 416,937 | 470,335 | 855,705 | 903,964 | 873,016 | |||||||||||
| Total debt | 8,380 | 8,417 | 10,612 | 340,759 | 325,294 | 510,622 | |||||||||||
| Shareholders' equity | 206,602 | 222,401 | 260,916 | 191,274 | 237,788 | 16,776 | |||||||||||
Retail sales data is referred to in "Management's Discussion and Analysis of Financial Condition and Results of Operations." Our use of retail sales reflect the fundamental role of "retail sales" in our accounting systems, internal controls and operations, including the basis upon which the distributors are being paid. In addition, information in daily and monthly reports reviewed by our management relies on retail sales data.
The following represents the reconciliation of retail sales to net sales for each of the periods set forth above:
| |
Year ended December 31, |
January 1 to July 31, |
August 1 to December 31, |
Year ended December 31, |
January 1 to March 31, |
January 1 to March 31, |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
1999 |
2000 |
2001 |
2002 |
2002 |
2003 |
2003 |
2004 |
|||||||||||||||||
| |
(predecessor) |
(predecessor) |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
(successor) |
(successor) |
|||||||||||||||||
| |
(dollars in thousands) |
||||||||||||||||||||||||
| Retail sales | $ | 1,793,508 | $ | 1,764,851 | $ | 1,656,168 | $ | 1,047,690 | $ | 731,505 | $ | 1,894,384 | $ | 457,073 | $ | 531,346 | |||||||||
| Distributor allowance | (837,283 | ) | (820,723 | ) | (774,513 | ) | (492,997 | ) | (345,145 | ) | (899,264 | ) | (216,675 | ) | (253,207 | ) | |||||||||
| Product sales | 956,225 | 944,128 | 881,655 | 554,693 | 386,360 | 995,120 | 240,398 | 278,139 | |||||||||||||||||
| Handling and freight income | 142,660 | 141,356 | 138,475 | 89,495 | 63,164 | 164,313 | 39,641 | 45,914 | |||||||||||||||||
| Net sales | $ | 1,098,885 | $ | 1,085,484 | $ | 1,020,130 | $ | 644,188 | $ | 449,524 | $ | 1,159,433 | $ | 280,039 | $ | 324,053 | |||||||||
42
acquisition. Adjusted EBITDA, as presented, may not be comparable to similarly titled measures reported by other companies.
The following table represents a reconciliation of net income to EBITDA and adjusted EBITDA:
| |
Year ended December 31, |
January 1 to July 31, |
August 1 to December 31, |
Year ended December 31, |
January 1 to March 31, |
January 1 to March 31, |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
1999 |
2000 |
2001 |
2002 |
2002 |
2003 |
2003 |
2004 |
|||||||||||||||||
| |
(predecessor) |
(predecessor) |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
(successor) |
(successor) |
|||||||||||||||||
| |
(dollars in thousands) |
||||||||||||||||||||||||
| Net income (loss) | $ | 56,923 | $ | 36,919 | $ | 42,588 | $ | 9,212 | $ | 14,005 | $ | 36,847 | $ | 16,870 | (485 | ) | |||||||||
| Minority interest | 1,086 | 1,058 | 725 | 189 | — | — | — | — | |||||||||||||||||
| Income taxes | 36,314 | 25,318 | 28,875 | 6,267 | 14,986 | 28,721 | 12,374 | 9,849 | |||||||||||||||||
| Interest (income) expense, net | (1,750 | ) | (2,354 | ) | (3,413 | ) | (1,364 | ) | 23,898 | 41,468 | 9,948 | 27,372 | |||||||||||||
| Depreciation & amortization | 14,001 | 15,693 | 18,056 | 11,722 | 11,424 | 55,605 | 6,389 | 11,406 | |||||||||||||||||
| EBITDA | 106,574 | 76,634 | 86,831 | 26,026 | 64,313 | 162,641 | 45,581 | 48,142 | |||||||||||||||||
| Merger transaction expenses | — | 9,498 | — | 54,708 | 6,183 | — | — | — | |||||||||||||||||
| Other(2) | — | — | — | — | — | 5,092 | 342 | — | |||||||||||||||||
| Adjusted EBITDA | $ | 106,574 | $ | 86,132 | $ | 86,831 | $ | 80,734 | $ | 70,496 | $ | 167,733 | $ | 45,923 | $ | 48,142 | |||||||||
43
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
The following unaudited pro forma condensed consolidated financial statements (the "pro forma condensed financial statements") are based on the historical financial statements of Holdings, included elsewhere herein, adjusted to give effect to the offering of the Outstanding Notes and the following: (i) the receipt of proceeds from the offering of the Outstanding Notes; (ii) the redemption of Holdings' Preferred Shares, including Preferred Shares that we expect to be issued in connection with the exercise of Holdings' Warrants, and payment of accrued but unpaid dividends; (iii) the purchase of Holdings' Senior Notes at a negotiated price; (iv) the application of available cash to reduce outstanding amounts under Herbalife's existing senior credit facilities; and (v) the payment of fees and expenses related to the aforementioned. All of the aforementioned items (i) to (v) are collectively referred to herein as the "Transactions." The pro forma condensed financial statements were prepared to illustrate the estimated effects of the Transactions. The unaudited pro forma condensed statements of income for the year ended December 31, 2003 and the three months ended March 31, 2004 give effect to the Transactions as if the transactions had occurred as of January 1, 2003. The pro forma adjustments are based upon available information and certain assumptions that Holdings believes are reasonable. The pro forma condensed financial statements do not purport to represent what Holdings' results of operations would actually have been had the Transactions in fact occurred as of the dates indicated above or to project Holdings' results of operations for these periods indicated or for any other period.
44
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF INCOME
For the Year Ended December 31, 2003
| |
December 31, 2003 |
Pro forma adjustments |
Pro forma |
|||||
|---|---|---|---|---|---|---|---|---|
| |
(dollars in thousands) |
|||||||
| Product sales | $ | 995,120 | $ | 995,120 | ||||
| Handling and freight income | 164,313 | 164,313 | ||||||
| Net sales | 1,159,433 | 1,159,433 | ||||||
| Cost of sales | 235,785 | 235,785 | ||||||
| Royalty overrides | 415,351 | 415,351 | ||||||
| Marketing, distribution, and administrative expenses | 401,261 | 401,261 | ||||||
| Interest expense, net | 41,468 | 18,094 | (1) | 59,562 | ||||
| Income before income taxes | 65,568 | 47,474 | ||||||
| Income taxes | 28,721 | 1,086 | (2) | 29,807 | ||||
| Net income | $ | 36,847 | $ | 17,667 | ||||
45
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF INCOME
For the Three Months Ended March 31, 2004
| |
March 31, 2004 |
Pro forma adjustments |
Pro forma |
|||
|---|---|---|---|---|---|---|
| |
(dollars in thousands) |
|||||
| Product sales | 278,139 | 278,139 | ||||
| Handling and freight income | 45,914 | 45,914 | ||||
| Net sales | 324,053 | 324,053 | ||||
| Cost of sales | 63,618 | 63,618 | ||||
| Royalty overrides | 115,857 | 115,857 | ||||
| Marketing, distribution, and administrative expenses | 107,842 | 107,842 | ||||
| Interest expense, net | 27,372 | (12,195) | (1) | 15,177 | ||
| Income before income taxes | 9,364 | 21,559 | ||||
| Income taxes | 9,849 | 326 | (2) | 10,175 | ||
| Net income | (485 | ) | 11,384 | |||
46
Notes to Unaudited Pro Forma Condensed Consolidated Financial Statements
(1) Interest Expense, Net:
The pro forma adjustments to interest expense are based on the amounts borrowed and the expected rates in effect at the closing of the Transactions:
| |
Year ended December 31, 2003 |
Three Months Ended March 31, 2004 |
||||||
|---|---|---|---|---|---|---|---|---|
| |
(dollars in millions) |
|||||||
| Elimination of historical interest: | ||||||||
| Interest expense on Holdings' Senior Notes | $ | 6.2 | $ | 16.5 | ||||
| Interest expense on Herbalife's term loan | 3.5 | 0.9 | ||||||
| Interest income on Holdings and Herbalife cash used in the transaction | (0.6 | ) | (0.1 | ) | ||||
| $ | 9.1 | $ | 17.3 | |||||
| Interest on the new borrowing: | ||||||||
| Interest expense on the Outstanding Notes | 26.1 | $ | 4.9 | |||||
| Amortization of discount and deferred financing costs on Outstanding Notes | 1.1 | 0.2 | ||||||
| Pro forma interest expense | 27.2 | $ | 5.1 | |||||
| Pro forma adjustment to interest expense net | $ | 18.1 | $ | (12.2 | ) | |||
Total transaction fees and costs, including those treated as debt discount, of $10.1 million were incurred related to the Outstanding Notes. Such costs will be amortized using the effective interest method, over the term of the related indebtedness. The term of the Outstanding Notes (and the New Notes) is 7 years.
The interest expense on Holdings' Senior Notes being eliminated for the three months ended March 31, 2004 includes $15.4 million charge resulting from the repurchase of these notes on March 8, 2004 and the write off of the associated discount and deferred financing costs.
(2) Income Taxes:
Holdings estimates that it will not be able to obtain a tax benefit for the additional interest expense from the Outstanding Notes or from the New Notes offered hereby, but expects to be taxed on the gain from the elimination of interest expense from the term loan and the interest income from Herbalife's cash used in this transaction. Holdings is currently evaluating alternatives to allow it to obtain full or partial tax benefit for the additional interest expense. The additional tax expense related to the elimination of interest expense is calculated as follows:
| |
Year ended December 31, 2003 |
Three Months Ended March 31, 2004 |
||||||
|---|---|---|---|---|---|---|---|---|
| |
(dollars in millions) |
|||||||
| Interest expense on Herbalife's term loan | $ | 3.3 | $ | 0.9 | ||||
| Interest income on Herbalife cash used in the transaction | (0.6 | ) | (0.1 | ) | ||||
| $ | 2.7 | $ | 0.8 | |||||
| Income tax rate | 39.7 | % | 37.0 | % | ||||
| Additional tax expenses | $ | 1.1 | $ | 0.3 | ||||
47
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
You should read the following discussion and analysis in conjunction with "Selected Consolidated Historical Financial Data," "Unaudited Pro Forma Condensed Consolidated Financial Statements" and the related notes and our consolidated financial statements and related notes, each included elsewhere in this prospectus.
General
As a result of the acquisition of Herbalife International, Inc. ("Herbalife") on July 31, 2002 by an investment group led by Whitney & Co., LLC and Golden Gate Private Equity, Inc., (the "Acquisition"), the audited financial statements included elsewhere herein consist of financial information from Herbalife and its subsidiaries (collectively, our "Predecessor") and Holdings and its subsidiaries (collectively, the "Successor," "we," "us," "our" or the "Company"). For the purpose of management's discussion and analysis of financial condition and results of operations, our results of operations, including our segment operations and cash flows for the year ended December 31, 2002 have been derived by combining the results of operations and cash flows of our Predecessor for the period starting January 1, 2002 through July 31, 2002 with the results of operations and cash flows of the Successor from August 1, 2002 through December 31, 2002. The results of operations and cash flows of our Predecessor prior to the Acquisition incorporated in the following discussion are the historical results and cash flows of our Predecessor. These results of our Predecessor do not reflect any purchase accounting adjustments, which are included in our results subsequent to the Acquisition. Due to the results of purchase accounting applied as a result of the Acquisition and the additional interest expense associated with the debt incurred to finance the Acquisition, our results of operations may not be comparable in all respects to the results of operations of our Predecessor prior to the Acquisition. However, our management believes a discussion of our 2002 operations is more meaningful by combining our results with the results of the Predecessor. The terms "we," "us," "our" and "Company" refer to our Predecessor before the Acquisition for periods through July 31, 2002 and to the Successor after the Acquisition for periods subsequent to July 31, 2002 or the entire year from January 1, 2002 to December 31, 2002 as the context requires.
We are a worldwide marketer of weight management products, nutritional supplements, and personal care products that support our customers' wellness and healthy lifestyles. We market and sell these products through a global network marketing organization comprised of over one million independent distributors in 58 countries.
"Retail sales" represent the gross sales amounts reflected on our invoices to our distributors. We do not receive the amount reported as "retail sales," and we do not monitor the actual retail prices charged for our products. "Product sales" represent the actual product purchase price paid to us by our distributors, after giving effect to distributor discounts, referred to as "distributor allowances," which total approximately 50% of suggested retail sales prices. Distributor allowances as a percentage of sales may vary by country depending upon regulatory restrictions that limit or otherwise restrict distributor allowances. "Net sales" represent product sales including handling and freight income. We utilize importers in a limited number of markets and, under some circumstances, we extend credit terms to these importers. Our "gross profit" consists of net sales less "cost of sales," consisting of the prices we pay to our manufacturers for products and costs related to product shipments, duties and tariffs, freight expenses relating to shipment of products to distributors and importers and similar expenses.
"Royalty overrides" consist of (i) royalty overrides and bonuses, which total approximately 15% and 7%, respectively, of the suggested retail sales prices of products earned by qualifying distributors on sales within their distributor organizations, (ii) the President's Team Bonus payable to some of our most senior distributors in the aggregate amount of approximately an additional 1% of product retail sales, and (iii) other discretionary incentive cash bonuses to qualifying distributors. These payments
48
generally represent compensation to distributors for the development and retention of the distributor sales organizations. Because of local country regulatory constraints, we may be required to modify our typical distributor incentive plans as described above. Consequently, the total distributor discount percentage may vary over time. We also offer reduced distributor allowances and pay reduced royalty overrides with respect to certain products worldwide.
Sales, related royalty overrides, and allowances for product returns are recorded when the merchandise is shipped in accordance with our shipping terms, which is when title passes. Advance sales deposits represent prepaid orders for which we have not shipped the merchandise.
Marketing, distribution and administrative expenses represent our operating expenses, components of which include labor and benefits, sales events, professional fees, travel and entertainment, advertising, occupancy costs, communication costs, bank fees, depreciation and amortization, foreign exchange fees and other miscellaneous operating expenses.
Most of our sales outside the United States are made in the respective local currencies. In preparing our financial statements, we translate revenues into U.S. dollars using average exchange rates. Additionally, the majority of our purchases from our suppliers generally are made in U.S. dollars, while sales to distributors generally are made in local currencies. Consequently, a strengthening of the U.S. dollar versus a foreign currency can have a negative impact on our reported sales and operating margins and can generate transaction losses on intercompany transactions. Throughout the last five years, foreign currency exchange rates have fluctuated significantly. From time to time, we enter into foreign exchange forward contracts and option contracts to mitigate our foreign currency exchange risk.
Results of Operations
Management believes that the growing focus on good health and increasing obesity throughout the world continue to provide an excellent opportunity for our weight management, inner nutrition and Outer Nutrition[nc_cad,176] products.
Our results of operations for the periods described below are not necessarily indicative of results of operations for future periods, which depend upon numerous factors, including our ability to attract and retain new distributors, further penetrate existing markets and introduce additional and new products into our markets.
Quarter ended March 31, 2004 Compared To Quarter ended March 31, 2003
For the three months ended March 31, 2004, net income decreased to ($0.5) million from $16.9 million in 2003. Net sales for the three months ended March 31, 2004, increased 15.7% to $324.1 million from $280.0 million in 2003 helped by the appreciation of foreign currencies, particularly the euro, in addition to increased volume in many markets within Europe and The Americas. The impact from increased sales was offset primarily by a $23.4 million increase in operating expenses resulting from a $5.5 million increase in amortization expense of intangibles, a $4.0 million unfavorable impact from appreciation in foreign currencies and a $7.2 million increase in labor costs, and $15.6 million of additional interest expense resulting from the recapitalization transaction completed in March 2004. Excluding the impact from the additional pre-tax amortization expense related to the Acquisition of $5.5 million and $15.6 million additional interest expense, income before tax in 2004 would have increased 4.5% compared to the same period in 2003. The improved result was attributed to increased sales in Europe, Brazil and Mexico partially offset by declines in Japan, South Korea, and the U.S. We expect our worldwide sales in 2004 to be higher than 2003.
49
Sales by Geographic Regions
| |
Three Months Ended March 31, |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2004 |
|
||||||||||||||||||||||||||||||
| (in millions) |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
% Change In Net Sales |
||||||||||||||||||||||
| The Americas | $ | 161.6 | $ | (77.0 | ) | $ | 84.6 | $ | 15.6 | $ | 100.2 | $ | 181.0 | $ | (86.6 | ) | $ | 94.4 | $ | 16.9 | $ | 111.3 | 11.1 | % | |||||||||
| Europe | 173.3 | (82.5 | ) | 90.8 | 15.1 | 105.9 | 223.6 | (106.8 | ) | 116.8 | 19.9 | 136.7 | 29.1 | % | |||||||||||||||||||
| Asia/Pacific Rim | 61.7 | (27.8 | ) | 33.9 | 4.5 | 38.4 | 75.5 | (34.8 | ) | 40.7 | 5.4 | 46.1 | 20.1 | % | |||||||||||||||||||
| Japan | 60.5 | (29.4 | ) | 31.1 | 4.4 | 35.5 | 51.2 | (24.9 | ) | 26.3 | 3.7 | 30.0 | (15.5 | )% | |||||||||||||||||||
| Total | $ | 457.1 | $ | (216.7 | ) | $ | 240.4 | $ | 39.6 | $ | 280.0 | $ | 531.3 | $ | (253.1 | ) | $ | 278.2 | $ | 45.9 | $ | 324.1 | 15.7 | % | |||||||||
Net sales in The Americas increased $11.1 million, or 11.1%, for the three months ended March 31, 2004 compared to the same period in 2003. In local currency, net sales increased by 7.7%. The increase was a result of sales growth in both Brazil and Mexico of $7.5 million and $4.5 million, respectively, partly offset by a $2.7 million decline in the U.S. During the months of February and March sales in the U.S. improved compared to last year. Our goal is to continue our efforts to revitalize the U.S. market through new product introductions, such as Shapeworks and Garden 7, enhance distributor business tools, open local sales centers and implement distributor leadership initiatives.
Net sales in Europe increased $30.8 million, or 29.1%, for the three months ended March 31, 2004 compared to the same period in 2003. In local currency, net sales increased 12.7% as compared to 2003. The appreciation of the euro and other European currencies was a major reason for the overall increase in net sales. Also, sales in many countries like Belgium, France, Netherlands, Portugal, Spain, Switzerland and Turkey recorded significant volume growth. In April of 2004, we strengthened our presence in Russia and Greece, expanding our distributor services by taking over the management of product distribution, which was previously handled through third party importers.
Net sales in Asia/Pacific Rim increased $7.7 million, or 20.1%, for the three months ended March 31, 2004 compared to the same period in 2003. In local currency, net sales increased 14.8%. The sales increase was due to a $5.2 million, or 49.9%, increase in Taiwan partly offset by a $3.4 million, or 28.7%, decrease in South Korea. During 2003, we implemented several new initiatives to help stem a sales decline by making improvements to distributor arrangements and, introducing new Internet tools and several new products. These initiatives have helped stabilize sales beginning in the second half of 2003 with net sales of approximately $9 million for each quarter. We expect South Korea's sales to remain at approximately this level.
Net sales in Japan decreased $5.5 million, or 15.5%, for the three months ended March 31, 2004 compared to the same period in 2003. In local currency, net sales in Japan decreased 19.4%. The decline in the Japanese market over the last four years has continued due to strong competition and limited product launches in Japan. In 2004, it is our continued goal to revitalize the Japanese market through new product introductions, enhancing distributor business tools, and implementing distributor leadership initiatives and training to rebuild a positive momentum.
50
Sales by Product Category
| |
Three Months Ended March 31, |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2003 |
2004 |
|
||||||||||||||||||||||||||||||
| (in millions) |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
% Change In Net Sales |
||||||||||||||||||||||
| Weight Management | $ | 200.7 | $ | (98.4 | ) | $ | 102.3 | $ | 17.4 | $ | 119.7 | $ | 239.0 | $ | (117.6 | ) | $ | 121.4 | $ | 20.7 | $ | 142.1 | 18.7 | % | |||||||||
| Inner Nutrition | 206.7 | (101.5 | ) | 105.2 | 17.9 | 123.1 | 234.5 | (115.4 | ) | 119.1 | 20.2 | 139.3 | 13.2 | % | |||||||||||||||||||
| Outer Nutrition® | 43.9 | (21.5 | ) | 22.4 | 3.8 | 26.2 | 49.9 | (24.6 | ) | 25.3 | 4.3 | 29.6 | 13.0 | % | |||||||||||||||||||
| Literature, Promotional and Other | 5.8 | 4.7 | 10.5 | 0.5 | 11.0 | 7.9 | 4.5 | 12.4 | 0.7 | 13.1 | 19.1 | % | |||||||||||||||||||||
| Total | $ | 457.1 | $ | (216.7 | ) | $ | 240.4 | $ | 39.6 | $ | 280.0 | $ | 531.3 | $ | (253.1 | ) | $ | 278.2 | $ | 45.9 | $ | 324.1 | 15.7 | % | |||||||||
For the three months ended March 31, 2004, net sales of weight management, inner nutrition and outer nutrition products increased 18.7%, 13.2% and 13.0%, respectively.
Gross Profit. Gross profit was $260.4 million for the three months ended March 31, 2004 compared to $223.1 million in the same period in 2003. As a percentage of net sales, gross profit for the period ended March 31, 2004 increased from 79.7% to 80.4% as compared to the prior period. The slight increase in gross profit reflected a reduction in the inventory provision for slow moving and anticipated obsolescence as a result of better inventory management, lower freight and duty expenses, and the favorable impact of stronger foreign currencies. We expect the gross margin to be maintained at approximately the current level for the remainder of 2004.
Royalty Overrides. Royalty overrides as a percentage of net sales were 35.8% for the three months ended March 31, 2004 as compared to 35.5% in the same period in 2003. The ratio varies slightly from period to period primarily due to changes in the mix of products and countries because full royalty overrides are not paid on certain products or in certain countries.
Marketing, Distribution, and Administrative Expenses. Marketing, distribution, and administrative expenses as a percentage of net sales were 33.3% for the three months ended March 31, 2004, as compared to 30.1% in the same period of 2003. For the three months ended March 31, 2004, these expenses increased $23.4 million to $107.8 million from $84.4 million in the same period in 2003. The increase included a $6.4 million amortization expense of intangibles in 2004 compared to $0.9 million in 2003. The increase was due to the final allocation of the purchase price in connection with the Acqusition during the third quarter of 2003. In addition, marketing, distribution, and administrative expenses were unfavorably impacted by the appreciation of foreign currencies of $4.0 million, higher labor costs of $4.6 million, a bonus relating to a refinancing transaction of $2.6 million, and higher promotional expenses of $2.9 million reflecting timing differences. We currently expect our 2004 marketing, distribution, and administrative expenses to increase approximately 5% over 2003, primarily due to the continued impact of the appreciation of foreign currencies and the timing of certain sales and marketing events.
Net Interest Expense. Net interest expense was $27.4 million for the three months ended March 31, 2004 as compared to $9.9 million in the same period in 2003. The increase was mainly due to the repurchase of the 15.5% Senior Notes in connection with the Transactions. We recorded net additional interest expense of $15.4 million related to the repurchase, which primarily represented the purchase premium and the write-off of discount and deferred financing costs associated with the repurchases of the 15.5% Senior Notes.
Income Taxes. Income taxes were $9.8 million for the three months ended March 31, 2004 as compared to $12.4 million for the same period in 2003. As a percentage of pre-tax income, the estimated effective income tax rate was 105.2% for the three months ended March 31, 2004 and 42.3% for the three months ended March 31, 2003. The increase in the effective tax rate was caused primarily by the lack of any tax benefit estimated for the interest expense described above related to the
51
repurchase of the 15.5% Senior Notes primarily resulting from the purchase premium and write-off of discount and deferred financing costs of $15.4 million, and the additional interest expense including amortization of discount and deferred financing costs associated with the Notes. The premium and write-off described in the preceeding sentence affected the first quarter provision. However, since these items are not going to recur in subsequent quarters, the effective income tax rate in the following three quarters is expected to drop to 46.9%, with the effective income tax rate for the full year of 2004 expected at 57.7%. We are currently evaluating alternatives to allow us to obtain full or partial tax benefit for the additional interest expense associated with the Notes.
Foreign Currency Fluctuations. Currency fluctuations had a favorable impact of $4.1 million on net income for the three months ended March 31, 2004 when compared to what current year net income would have been using last year's foreign exchange rates. For the three months ended March 31, 2004, the regional effects were a favorable $0.7 million in The Americas, an unfavorable $0.9 million in Japan, and a favorable $3.4 million in Europe, with no material impact in the Pacific Rim.
Year ended December 31, 2003 compared to year ended December 31, 2002
For the year ended December 31, 2003, net income increased to $36.8 million from $23.2 million in 2002. Net sales for the for the year ended December 31, 2003, increased 6% to $1,159.4 million from $1,093.7 million in 2002 helped by the appreciation of foreign currencies and in particular the euro. Excluding the impact of pre-tax amortization expense of intangibles resulting from the Acquisition of $34.5 million and $1.5 million in 2003 and 2002, respectively, transaction expenses of $60.9 million in 2002 relating to the Acquisition, 2003 legal and related costs associated with litigation resulting from the Acquisition of $5.1 million, $6.2 million in incremental fees and expenses paid to our Equity Sponsors in 2003, and the favorable impact of foreign currency appreciation of approximately $15.8 million in 2003, operating income increased 5.7% to $137.0 million in 2003 from $129.6 million in 2002. The improved result was attributed to increased sales throughout Europe, Brazil and Mexico partly offset by the decreased sales in the U.S., Japan and South Korea. We expect that sales in the U.S., Japan and South Korea will improve following the execution of our revitalization initiatives for 2004, which are described below. We anticipate some impact associated with the discovery of Bovine Spongiform Encephalopathy ("BSE"), a disease commonly referred to as "mad cow disease," in the United States, but do not expect this issue to have a material effect on our business.
Sales by Geographic Regions
| |
Year Ended December 31, |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
|||||||||||||||||||||||||||||||
| |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
% Change in Net Sales |
||||||||||||||||||||||
| |
(dollars in millions) |
||||||||||||||||||||||||||||||||
| The Americas | $ | 683.1 | $ | (324.7 | ) | $ | 358.4 | $ | 65.9 | $ | 424.3 | $ | 687.9 | $ | (328.9 | ) | $ | 359.0 | $ | 65.4 | $ | 424.4 | — | ||||||||||
| Europe | 560.3 | (266.3 | ) | 294.0 | 48.7 | 342.7 | 733.4 | (349.4 | ) | 384.0 | 64.2 | 448.2 | 30.8 | % | |||||||||||||||||||
| Asia/Pacific Rim | 294.7 | (130.0 | ) | 164.7 | 20.8 | 185.5 | 271.6 | (123.6 | ) | 148.0 | 19.5 | 167.5 | -9.7 | % | |||||||||||||||||||
| Japan | 241.1 | (117.1 | ) | 124.0 | 17.2 | 141.2 | 201.5 | (97.4 | ) | 104.1 | 15.2 | 119.3 | -15.5 | % | |||||||||||||||||||
| Total | $ | 1,779.2 | $ | (838.1 | ) | $ | 941.1 | $ | 152.6 | $ | 1,093.7 | $ | 1,894.4 | $ | (899.3 | ) | $ | 995.1 | $ | 164.3 | $ | 1,159.4 | 6.0 | % | |||||||||
Net sales in The Americas remained flat with 2002. In local currency, net sales increased by 1.9%. The slight increase was a result of increases in both Brazil and Mexico, which were mostly offset by declining sales in the U.S. Net sales in Brazil and Mexico increased 71.4% and 13.3%, respectively, while net sales in the U.S. declined 10.3% in 2003. In the fourth quarter of 2003, the sales decline in the U.S. slowed in connection with the introduction of a new sales promotion. In 2004, it is our goal to
52
revitalize the U.S. market through new product introductions, the enhanced use of internet tools, the opening of strategically located sales centers, and the implementation of distributor leadership initiatives to rebuild a positive momentum.
Net sales in Europe increased $105.5 million or 30.8% in 2003 compared to the prior year. In local currency, net sales increased 14.7% as compared to 2002. The appreciation of the euro and other European currencies was a primary reason for the overall sales increase, but sales in many of the established countries like Belgium, France, Netherlands, Spain, Switzerland and Turkey had notable volume growth. In 2004, it is our goal to strengthen our presence in Europe and in particular in Russia and Greece by expanding our distributor services and taking over the management of product distribution, which in the past has been handled through third party importers.
Net sales in Asia/Pacific Rim decreased $18.0 million or 9.7% in 2003 as compared to the prior year. In local currency, net sales decreased 13.3%. The sales decrease was due to a $32.5 million or 42.5% decline in South Korea partly offset by a $9.6 million or 25% increase in Taiwan. During 2003 we implemented several new initiatives to help the distributors in South Korea regain momentum, including improving their incentive arrangements and introducing new internet tools and several new products. We believe that these initiatives have helped stabilize sales during the second half of 2003.
Net sales in Japan decreased $21.9 million, or 15.5% during 2003 as compared to the prior year. In local currency, net sales in Japan decreased 22.8%. The decline in the Japanese market over the last year has continued due to strong competition and the general deterioration in economic conditions in Japan. In 2004, it is our goal to revitalize the Japanese market through new product introductions, enhanced use of internet tools, and the implementation of distributor leadership initiatives and training to rebuild positive momentum.
Sales by Product Category
| |
Year Ended December 31, |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
|||||||||||||||||||||||||||||||
| |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
% Change in Net Sales |
||||||||||||||||||||||
| |
(dollars in millions) |
||||||||||||||||||||||||||||||||
| Weight Management | $ | 779.8 | $ | (381.1 | ) | $ | 398.7 | $ | 66.9 | $ | 465.6 | $ | 840.4 | $ | (413.2 | ) | $ | 427.2 | $ | 72.9 | $ | 500.1 | 7.4 | % | |||||||||
| Inner nutrition | 797.7 | (389.8 | ) | 407.9 | 68.4 | 476.3 | 849.0 | (417.5 | ) | 431.5 | 73.6 | 505.1 | 6.0 | % | |||||||||||||||||||
| Outer Nutrition® | 182.0 | (88.9 | ) | 93.1 | 15.6 | 108.7 | 177.6 | (87.3 | ) | 90.3 | 15.4 | 105.7 | -2.8 | % | |||||||||||||||||||
| Literature, Promotional and Other | 19.7 | 21.7 | 41.4 | 1.7 | 43.1 | 27.4 | 18.7 | 46.1 | 2.4 | 48.5 | 12.5 | % | |||||||||||||||||||||
| Total | $ | 1,779.2 | $ | (838.1 | ) | $ | 941.1 | $ | 152.6 | $ | 1,093.7 | $ | 1,894.4 | $ | (899.3 | ) | $ | 995.1 | $ | 164.3 | $ | 1,159.4 | 6.0 | % | |||||||||
For the year ended December 31, 2003, net sales of weight management and inner nutrition products increased 7.4% and 6.0%, respectively, while Outer Nutrition® declined 2.8% as compared to the prior year. We have over the last year increased our emphasis on science-based products and have contributed to, and helped fund, the UCLA Lab. During 2002 we rationalized our Outer Nutrition® line by eliminating color cosmetics. We believe that our Outer Nutrition® product line is now better aligned with our other product categories.
Gross Profit. Gross profit was $923.6 million for the year ended December 31, 2003 compared to $858.2 million in the prior year. As a percentage of net sales, gross profit for the year ended December 31, 2003 increased from 78.5% to 79.7% as compared to the prior year. The increase in gross profit reflected a reduction in the inventory provision for slow moving and anticipated obsolescence as a result of better inventory management, lower freight and duty expenses, and the favorable impact of stronger foreign currencies.
Royalty Overrides. Royalty overrides as a percentage of net sales were 35.8% for the year ended December 31, 2003 as compared to 35.4% in the prior year. The ratio varies slightly from period to
53
period primarily due to a change in the mix of products and countries because full royalty overrides are not paid on certain products or in certain countries.
Marketing, Distribution and Administrative Expenses. Marketing, distribution and administrative expenses as a percentage of net sales were 34.6% for the year ended December 31, 2003, as compared to 31.4% in the prior year. For the year ended December 31, 2003, these expenses increased $58.4 million to $401.3 million from $342.9 million in the prior year. The increase included $34.5 million amortization expense of intangibles in 2003 compared to $1.5 million in 2002. In addition, marketing, distribution and administrative expenses were unfavorably impacted by approximately $10.9 million due to the appreciation of foreign currencies, by approximately $6.9 million due to increased promotional expenses, by approximately $9.1 million due to litigation costs and related legal expenses, and by approximately $6.2 million due to fees and expenses paid to our Equity Sponsors subsequent to the Acquisition. Lower labor costs partly offset the increased expense reflecting efficiencies realized from various cost savings initiatives. We currently expect our 2004 marketing, distribution and administrative expenses to be flat with 2003.
Merger Transaction Expenses. In 2002, we recorded $21.9 million relating to fees and $39.0 million of stock option expenses in connection with the Acquisition.
Net Interest Expense. Net interest expense was $41.5 million for the year ended December 31, 2003 as compared to $22.5 million in the prior year. The increase was mainly due to a full year's interest expense relating to the term loan, Herbalife's senior subordinated notes and Holdings' Senior Notes in 2003 as compared to only five months of interest expense for those same items in 2002.
Income Taxes. Income taxes were $28.7 million for the year ended December 31, 2003 as compared to $21.3 million for the prior year. As a percentage of pre-tax income, the estimated annual effective income tax rate was 43.8% for 2003 and 47.6% for 2002. The decrease in effective tax rate reflected primarily the acquisition-related expenses incurred by Holdings in 2002.
Foreign Currency Fluctuations. Currency fluctuations had a favorable impact of $9.5 million on net income for the year ended December 31, 2003 when compared to what current year net income would have been using last year's foreign exchange rates. For the year ended December 31, 2003, the regional effects were $(3.2) million in The Americas, $1.5 million in the Pacific Rim, $11.2 million in Europe, and no material impact in Japan.
Net Income. Net income for the year ended December 31, 2003 was $36.8 million compared to net income of $23.2 million for the prior year. Net income increased primarily because of the factors noted above.
Year ended December 31, 2002 compared to year ended December 31, 2001
Net sales for year ended December 31, 2002 increased 7.2% to $1,093.7 million, as compared to net sales of $1,020.1 million in the prior year.
Sales by Geographical Regions
| |
Year Ended December 31, |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2001 |
2002 |
|||||||||||||||||||||||||||||||
| |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
% Change in Net Sales |
||||||||||||||||||||||
| |
(dollars in millions) |
||||||||||||||||||||||||||||||||
| Americas | $ | 620.2 | $ | (291.9 | ) | $ | 328.3 | $ | 58.6 | $ | 386.9 | $ | 683.1 | $ | (324.7 | ) | $ | 358.4 | $ | 65.9 | $ | 424.3 | 9.7 | % | |||||||||
| Europe | 459.5 | (216.1 | ) | 243.4 | 39.8 | 283.2 | 560.3 | (266.3 | ) | 294.0 | 48.7 | 342.7 | 21.0 | % | |||||||||||||||||||
| Asia/Pacific Rim | 271.9 | (118.9 | ) | 153.0 | 19.0 | 172.0 | 294.7 | (130.0 | ) | 164.7 | 20.8 | 185.5 | 7.8 | % | |||||||||||||||||||
| Japan | 304.6 | (147.6 | ) | 157.0 | 21.0 | 178.0 | 241.1 | (117.1 | ) | 124.0 | 17.2 | 141.2 | -20.7 | % | |||||||||||||||||||
| Total | $ | 1,656.2 | $ | (774.5 | ) | $ | 881.7 | $ | 138.4 | $ | 1,020.1 | $ | 1,779.2 | $ | (838.1 | ) | $ | 941.1 | $ | 152.6 | $ | 1,093.7 | 7.2 | % | |||||||||
54
Net sales in The Americas increased $37.4 million or 9.7% as compared to the prior year. In local currency, net sales increased by 13.7%. The increase was mainly due to well-organized distributor sales meetings, and strong local leadership.
Net sales in Europe increased $59.5 million or 21.0% in 2002 as compared to the prior year. In local currency, net sales in Europe increased 14.6%. The increase was partly due to strong local distributor leadership and effective lead generation system.
Net sales in Asia/Pacific Rim increased $13.5 million or 7.8% during 2002 as compared to the prior year. In local currency, net sales for Asia/Pacific Rim increased 5.8%. The increase was due to sales growth in Australia, Taiwan and Thailand of 39.9%, 11.1% and 76.1%, respectively.
Net sales in Japan decreased $36.8 million, or 20.7% during 2002 as compared to the prior year. In local currency, net sales for Japan decreased 18.3%. The decline was due to deteriorating economic conditions and the intensified competitive sales environment.
Sales by Product Category
| |
Year Ended December 31, |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2001 |
2002 |
|||||||||||||||||||||||||||||||
| |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
Retail Sales |
Distributor Allowance |
Product Sales |
Handling & Freight Income |
Net Sales |
% Change in Net Sales |
||||||||||||||||||||||
| |
(dollars in millions) |
||||||||||||||||||||||||||||||||
| Weight Management | $ | 707.9 | $ | (345.2 | ) | $ | 362.7 | $ | 59.2 | $ | 421.9 | $ | 779.8 | $ | (381.1 | ) | $ | 398.7 | $ | 66.9 | $ | 465.6 | 10.4 | % | |||||||||
| Inner Nutrition | 744.6 | (363.1 | ) | 381.5 | 62.2 | 443.7 | 797.7 | (389.8 | ) | 407.9 | 68.4 | 476.3 | 7.3 | % | |||||||||||||||||||
| Outer Nutrition® | 178.2 | (86.9 | ) | 91.3 | 14.9 | 106.2 | 182.0 | (88.9 | ) | 93.1 | 15.6 | 108.7 | 2.4 | % | |||||||||||||||||||
| Literature, Promotional and Other | 25.5 | 20.7 | 46.2 | 2.1 | 48.3 | 19.7 | 21.7 | 41.4 | 1.7 | 43.1 | -10.8 | % | |||||||||||||||||||||
| Total | $ | 1,656.2 | $ | (774.5 | ) | $ | 881.7 | $ | 138.4 | $ | 1,020.1 | $ | 1,779.2 | $ | (838.1 | ) | $ | 941.1 | $ | 152.6 | $ | 1,093.7 | 7.2 | % | |||||||||
For the year ended December 31, 2002, net sales of weight management, inner nutrition and Outer Nutrition® products increased as compared to the prior year. The increases were partially offset by a decrease in sales of literature, promotional and other materials and an increase in returns and refunds.
Gross Profit. Gross profit was $858.2 million for the year ended December 31, 2002 compared to $778.6 million in the prior year. As a percentage of net sales, gross profit for the year ended December 31, 2002 increased from 76.3% to 78.5% as compared to the prior year. The increase in gross profit reflected the realization of product cost savings attributed to new supply contracts initiated in 2001 and a reduction in the inventory provision for slow moving and anticipated obsolescence when comparing 2002 to 2001.
Royalty Overrides. Royalty overrides as a percentage of net sales were 35.4% for the year ended December 31, 2002 as compared to 34.8% in the prior year. The ratio varies slightly from period to period primarily due to a change in the mix of products and countries because full royalty overrides are not paid on certain products or in certain countries.
Marketing, Distribution and Administrative Expenses. Marketing, distribution and administrative expenses as a percentage of net sales were 31.4% for the year ended December 31, 2002, as compared to 34.8% in the prior year. For the year ended December 31, 2002, these expenses decreased $11.7 million to $342.9 million from $354.6 million in the prior year. The decrease was due to $9.3 million in charges for non-income tax contingencies for various tax audits in 2001, a $5.4 million decrease in severance expense from 2001 to 2002, partially offset by $1.3 million higher foreign exchange losses in 2002.
Merger Transaction Expenses. In 2002, we recorded $21.9 million relating to fees and $39.0 million of stock option expenses in connection with the Acquisition.
55
Net Interest Expense. Net interest expense was $22.5 million for the year ended December 31, 2002 as compared to net interest income of $3.4 million in the prior year. In 2002, the interest expense was mainly related to the term loan, Holdings' Senior Notes and the senior subordinated notes issued to finance the Acquisition.
Income Taxes. Income taxes were $21.3 million for the year ended December 31, 2002 as compared to $28.9 million for the prior year. As a percentage of pre-tax income, the estimated annual effective income tax rate was 47.6% and 40% for 2002 and 2001, respectively.
Foreign Currency Fluctuations. Currency fluctuations had an unfavorable effect of $1.0 million on net income for the year ended December 31, 2002 when recalculating current year net income using last year's foreign exchange rates. For the year ended December 31, 2002, the regional effects were $3.2 million unfavorable in The Americas, $1.1 million unfavorable in the Pacific Rim, and $3.3 million favorable in Europe.
Net Income. Net income for the year ended December 31, 2002 was $23.2 million compared to net income of $42.6 million for the prior year. Excluding the impact of Acquisition expenses, amortization of intangibles and changes in net interest expense, net income for the year ended December 31, 2002 would have been $76.2 million. Net income excluding the impact of Acquisition expenses for the year ended December 31, 2002 increased principally because of a 7.2% increase in net sales and a 2.1% increase in gross profit as a percentage of net sales.
Holdings' Liquidity and Capital Resources
We have historically met our working capital and capital expenditure requirements, including funding for expansion of operations, through net cash flows provided by operating activities. Our principal source of liquidity is our operating cash flows. A substantial decrease in sales of our products would reduce the availability of funds.
For the year ended December 31, 2003, net cash provided by operating activities was $94.6 million compared to $65.9 million in the prior year. The increase in cash generated from operating activities was primarily related to the increase in net income adjusted for non cash items of $54.9 million partially offset by a decrease in working capital of $26.2 million.
For the three months ended March 31, 2004, we generated $25.7 million from operating cash flows compared to the use of $3.5 million in the same period in 2003. The increase in cash generated from operations is primarily related to changes in working capital which were primarily due to payments of severance, deferred compensation liability and debt interests.
Capital expenditures including acquisitions of property under capitalized leases for the year ended December 31, 2003 were $20.4 million compared to $10.4 million in the prior year. The majority of the 2003 capital expenditures resulted from investment in management information systems, office facilities and equipment in the United States.
Capital expenditures including capital leases for the three months ended March 31, 2004 were $5.4 million compared to $2.7 million in the same period in 2003. The majority of these expenditures represented investments in management information systems, office facilities and equipment in the United States. In 2004, we expect to incur approximately $40 million in similar types of investments. These investments will be primarily centered around our internet, order management, and inventory systems. It is our expectation that these investments will result in additional distributor recruiting, retention, and retailing activities which we expect ultimately will drive additional revenue generation. As currently contemplated, the projected cost in 2004 for these new investments will be between $20 million and $25 million. Accordingly, we anticipate that we will incur up to $40 million in capital expenditures for fiscal year 2004.
56
As of December 31, 2003, we had $1.5 million in working capital. Cash, cash equivalents and marketable securities, including restricted cash, were $156.4 million at December 31, 2003, compared to $76.0 million at December 31, 2002.
As of March 31, 2004, we had working capital of $18.5 million. Cash and cash equivalents were $123.0 million at March 31, 2004, compared to $150.7 million at December 31, 2003. Our cash, in addition to liquidity provided from future operating cash flows and a revolving credit facility of $25 million, are expected to be sufficient to meet our working capital requirements for the foreseeable future. During the first quarter of our fiscal year, we generally make annual payments including certain distributor bonuses, which amount to approximately 1% of annual retail sales. In addition, on January 15th and July 15th of each year, we make coupon payments on Herbalife's 113/4% Senior Subordinated Notes due 2010, which amount to approximately $9.4 million.
In connection with the Acquisition, we consummated certain related financing transactions, which included Herbalife's issuance of its 113/4% Senior Subordinated Notes due 2010 in the amount of $165 million, Holdings' issuance of the Senior Notes in the amount of $38 million and the entering into of Herbalife's senior credit facilities, consisting of a term loan in the amount of $180 million and a revolving credit facility in the amount of $25 million.
In March 2004, we and our lenders amended our Credit Agreement. Under the terms of the amendment, we made a prepayment of $40.0 million. In connection with this prepayment, our lenders waived the March 31, 2004 mandatory amortization payment of $6.5 million along with a mandatory 50% excess cash flow payment for the year ended December 31, 2003. The amendment also included a lower interest rate margin, increased capital spending allowance and the ability for our parent company to complete a recapitalization. Our debt agreement has a provision that requires us to make annual payments to the extent of excess cash flow, as defined. The schedule of the principal payments was also modified so that we were obligated to pay approximately $4.4 million on March 31, 2004 and in each subsequent quarter through June 30, 2008.
In March 2004, Holdings and its wholly-owned subsidiary WH Capital Corporation completed the $275 million offering of the Outstanding Notes. The proceeds of the offering together with available cash were used to pay the cash redemption price due upon redemption of all outstanding Holdings convertible Preferred Shares, including all accrued and unpaid dividends, to redeem Holdings' 15.5% Senior Notes, and to pay related fees and expenses. Interest on the Outstanding Notes will be paid in cash semi-annually in arrear on April 1 and October 1 of each year, starting on October 1, 2004. The Outstanding Notes are Holdings' general unsecured obligations, ranking equally with any of the existing and future senior indebtedness and senior to all of Holdings' future subordinated indebtedness. Also, the Outstanding Notes are effectively subordinated to all existing and future indebtedness and other liabilities of Holdings' subsidiaries.
For further discussion of the financings, see Note 4 in the Notes to the Consolidated Financial Statements contained herein and "Risk Factors—Risks Relating to the Notes."
The following summarizes our contractual obligations at December 31, 2003, and the effect such obligations are expected to have on our liquidity and cash flow in future periods.
| |
Payments Due |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total |
Less than 1 year |
1 - 2 years |
3 - 5 years |
More than 5 years |
||||||||||
| |
(dollars in millions) |
||||||||||||||
| Long Term Debt(1) | $ | 319.8 | $ | 69.2 | $ | 15.7 | $ | 38.7 | $ | 196.2 | |||||
| Capital Leases(1)(2) | 5.7 | 3.3 | 1.9 | 0.5 | — | ||||||||||
| Operating Leases(2) | 29.7 | 12.6 | 9.4 | 7.3 | 0.4 | ||||||||||
| Total | $ | 355.2 | $ | 85.1 | $ | 27.0 | $ | 46.5 | $ | 196.6 | |||||
57
In addition to the commitments noted above, we have several retirement plans for which we have recorded a long-term compensation liability of $22.4 million as of December 31, 2003. We have established an irrevocable trust whose assets are not intended to be reachable by our creditors, and a "rabbi trust" whose assets will be used to pay the benefits if we remain solvent, but can be reached by our creditors if we become insolvent. The values of the assets in the irrevocable trust and the "rabbi trust" were $2.8 million and $18.5 million, respectively, as of December 31, 2003. Please see Notes 6 and 7 in the Notes to Consolidated Financial Statements for a description of these plans. As these amounts will be paid to employees upon retirement or in some cases upon termination of employment, the period in which these benefits will be paid cannot be determined.
We also have contingent liabilities related to legal proceedings and other matters arising in the ordinary course of business. Although it is reasonably possible we may incur losses upon conclusion of such matters, an estimate of any loss or range of loss cannot be made. In the opinion of management, it is expected that amounts, if any, which may be required to satisfy such contingencies will not have a material impact on our consolidated financial statements.
Historically, we have not been subjected to material price increases by our suppliers. We believe that in the event of price increases, we have the ability to respond to a portion of any price increases by raising the price of our products. The majority of our purchases from suppliers are generally made in U.S. dollars, while sales to Herbalife distributors generally are made in local currencies. Consequently, strengthening of the U.S. dollar versus a foreign currency can have a negative impact on operating margins and can generate transaction losses on intercompany transactions. For discussion of our foreign exchange contracts and other hedging arrangements, see the quantitative and qualitative disclosures about market risks described below.
As a result of the Transactions and the Outstanding Notes offering, we have incurred significant debt service payments, including interest due in future years. It is estimated that total pro forma cash interest expense would have been approximately $53.0 million in 2003 if amounts under the Outstanding Notes were outstanding for the full year. No principal payment on the Outstanding Notes (or the New Notes) will be required until April 2011.
Our senior credit facilities impose certain restrictions on our ability to make capital expenditures. The indenture governing the Notes and our amended and restated senior credit facilities restrict, among other things, our ability to borrow money, pay dividends on or repurchase capital stock, make investments and sell assets or enter into mergers or consolidations. For more information, see "Description of Other Indebtedness," "Description of Notes" and "Risk Factors."
We expect that funds provided from operations and available borrowings under our revolving credit facility will provide sufficient working capital to operate our business, make expected capital expenditures and meet foreseeable liquidity requirements, including debt service on the Notes and the senior credit facilities. There can be no assurance, however, that our business will generate sufficient cash flows or that future borrowings will be available in an amount sufficient to enable us to service our debt, including the Notes, or to fund our other liquidity needs. See "Risk Factors."
For a discussion of certain contingencies that may impact liquidity and capital resources, see Note 9 in the Notes to Consolidated Financial Statements included herein.
Quantitative and Qualitative Disclosures About Market Risks
We are exposed to market risks, which arise during the normal course of business from changes in interest rates and foreign currency exchange rates. On a selected basis, we use derivative financial instruments to manage or hedge these risks. All hedging transactions are authorized and executed pursuant to written guidelines and procedures.
We have adopted Statement of Financial Accounting Standards No. ("SFAS") 133, "Accounting for Derivative Instruments and Hedging Activities." SFAS 133, as amended and interpreted, established
58
accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and for hedging activities. All derivatives, whether designated in hedging relationships or not, are required to be recorded on the balance sheet at fair value. If the derivative is designated as a fair-value hedge, the changes in the fair value of the derivative and the underlying hedged item are recognized concurrently in earnings. If the derivative is designated as a cash-flow hedge, changes in the fair value of the derivative are recorded in other comprehensive income ("OCI") and are recognized in the statement of operations when the hedged item affects earnings. SFAS 133 defined new requirements for designation and documentation of hedging relationships as well as ongoing effectiveness assessments in order to use hedge accounting. For a derivative that does not qualify as a hedge, changes in fair value are recognized concurrently in earnings.
A discussion of our primary market risk exposures and derivatives is presented below.
Foreign Exchange Risk
We enter into foreign exchange derivatives in the ordinary course of business primarily to reduce exposure to currency fluctuations attributable to intercompany transactions and translation of local currency revenue. Most of these foreign exchange contracts are designated for forecasted transactions.
We purchase average rate put options, which give us the right, but not the obligation, to sell foreign currency at a specified exchange rate ("strike rate"). These contracts provide protection in the event the foreign currency weakens beyond the option strike rate. In some instances, we sell (write) foreign currency call options to finance the purchase of put options, which gives the counterparty the right, but not the obligation, to buy foreign currency from us at a specified strike rate. These contracts serve to limit the benefit we would otherwise derive from strengthening of the foreign currency beyond the strike rate. Such written call options are only entered into contemporaneously with purchased put options. The fair value of option contracts is based on third-party bank quotes.
The following table provides information about the details of our option contracts at March 31, 2004.
| Foreign Currency |
Coverage |
Average Strike Price |
Fair Value |
Maturity Date |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
|
(in millions) |
|
||||||
| At March 31, 2004 | ||||||||||
| Purchased Puts (Company may Sell yen/Buy USD) | ||||||||||
| Japanese yen | $ | 10.5 | 103.65-107.62 | $ | 0.1 | Apr-Jun 2004 | ||||
| Japanese yen | $ | 10.5 | 103.34-107.25 | $ | 0.2 | July-Sep 2004 | ||||
| Japanese yen | $ | 10.5 | 102.98-106.80 | $ | 0.2 | Oct-Dec 2004 | ||||
Purchased Puts (Company may Sell euro/Buy USD) |
||||||||||
| Euro | $ | 9.2 | 1.1588-1.1881 | $ | — | Apr-Jun 2004 | ||||
| Euro | $ | 5.5 | 1.1564-1.1579 | $ | — | Jul-Sep 2004 | ||||
| Euro | $ | 5.5 | 1.1550-1.1558 | $ | 0.1 | Oct-Dec 2004 | ||||
59
The following table provides information about the details of our option contracts at December 31, 2003 and December 31, 2002:
| Foreign Currency |
Coverage |
Average Strike Price |
Fair Value |
Maturity Date |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
|
(in millions) |
|
||||||
| At December 31, 2003 | ||||||||||
| Purchased Puts (Company may Sell yen/Buy USD) | ||||||||||
Japanese yen |
$ |
6.0 |
107.75 - 107.97 |
— |
Jan - Mar 2004 |
|||||
| Japanese yen | $ | 6.0 | 107.39 - 107.62 | $ | 0.1 | Apr - Jun 2004 | ||||
| Japanese yen | $ | 6.0 | 106.95 - 107.25 | $ | 0.2 | Jul - Sep 2004 | ||||
| Japanese yen | $ | 6.0 | 106.43 - 106.80 | $ | 0.2 | Oct - Dec 2004 | ||||
| $ | 24.0 | $ | 0.5 | |||||||
Written Calls (Counterparty may Buy yen/Sell USD) |
||||||||||
Japanese yen |
$ |
6.0 |
102.00 |
— |
Jan - Mar 2004 |
|||||
| Japanese yen | $ | 6.0 | 102.00 | — | Apr - Jun 2004 | |||||
| Japanese yen | $ | 6.0 | 102.00 | $ | (0.1 | ) | Jul - Sep 2004 | |||
| Japanese yen | $ | 6.0 | 102.00 | $ | (0.1 | ) | Oct - Dec 2004 | |||
| $ | 24.0 | $ | (0.2 | ) | ||||||
Purchased Puts (Company may Sell euro/Buy USD) |
||||||||||
Euro |
$ |
9.4 |
1.1635 - 1.1910 |
— |
Jan - Mar 2004 |
|||||
| Euro | $ | 9.4 | 1.588 - 1.1881 | $ | 0.1 | Apr - Jun 2004 | ||||
| Euro | $ | 5.7 | 1.1564 - 1.1579 | — | Jul - Sep 2004 | |||||
| Euro | $ | 5.7 | 1.150 - 1.1558 | $ | 0.1 | Oct - Dec 2004 | ||||
| $ | 30.2 | $ | 0.2 | |||||||
Written Calls (Counterparty may Buy euro/Sell USD) |
||||||||||
Euro |
$ |
5.7 |
1.2100 |
$ |
(0.2 |
) |
Jan - Mar 2004 |
|||
| Euro | $ | 5.7 | 1.2100 | $ | (0.3 | ) | Apr - Jun 2004 | |||
| Euro | $ | 5.7 | 1.2100 | $ | (0.3 | ) | Jul - Sep 2004 | |||
| Euro | $ | 5.7 | 1.2100 | $ | (0.3 | ) | Oct - Dec 2004 | |||
| $ | 22.8 | $ | (1.1 | ) | ||||||
| At December 31, 2002 | ||||||||||
| Purchased Puts (Company may Sell yen/Buy USD) | ||||||||||
Japanese yen |
$ |
6.0 |
118.43 - 119.68 |
$ |
0.1 |
Jan - Mar 2003 |
||||
| Japanese yen | $ | 6.0 | 118.03 - 119.30 | $ | 0.1 | Apr - Jun 2003 | ||||
| $ | 12.0 | $ | 0.2 | |||||||
Purchased Puts (Company may Sell euro/Buy USD) |
||||||||||
Euro |
$ |
9.0 |
1.0155 - 1.0300 |
— |
Jan - Mar 2003 |
|||||
| Euro | $ | 9.0 | 1.0155 - 1.0300 | $ | 0.2 | Apr - Jun 2003 | ||||
| $ | 18.0 | $ | 0.2 | |||||||
60
Foreign exchange forward contracts are occasionally used to hedge advances between subsidiaries and bank loans denominated in currencies other than their local currency. The objective of these contracts is to neutralize the impact of foreign currency movements on the subsidiary's operating results. The fair value of forward contracts is based on third-party bank quotes.
The following table provides information about the details of our forward contracts at March 31, 2004.
| Foreign Currency |
Contract Date |
Forward Position |
Maturity Date |
Contract Rate |
Fair Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
(in millions) |
|
|
(in millions) |
|||||||
| At March 31, 2004 | ||||||||||||
| Buy TWD Sell EURO | 3/1/2004 | $ | 2.5 | 4/1/2004 | 41.3670 | $ | 2.6 | |||||
| Buy TWD Sell EURO | 3/1/2004 | $ | 1.0 | 4/1/2004 | 41.2750 | $ | 1.0 | |||||
| Buy CAD Sell EURO | 3/1/2004 | $ | 1.1 | 4/1/2004 | 1.6651 | $ | 1.1 | |||||
| Buy DKK Sell EURO | 3/1/2004 | $ | 0.8 | 4/1/2004 | 7.4503 | $ | 0.8 | |||||
| Buy AUD Sell EURO | 3/1/2004 | $ | 3.8 | 4/1/2004 | 1.6142 | $ | 3.8 | |||||
| Buy SEK Sell EURO | 3/1/2004 | $ | 0.8 | 4/1/2004 | 9.2429 | $ | 0.8 | |||||
| Buy NOK Sell EURO | 3/1/2004 | $ | 0.8 | 4/1/2004 | 8.6800 | $ | 0.9 | |||||
| Buy GBP Sell USD | 3/22/2004 | $ | 3.3 | 4/23/2004 | 1.8440 | $ | 3.3 | |||||
| Buy SEK Sell USD | 3/22/2004 | $ | 1.6 | 4/23/2004 | 7.4582 | $ | 1.6 | |||||
| Buy JPY Sell USD | 3/22/2004 | $ | 18.8 | 4/23/2004 | 106.8000 | $ | 19.3 | |||||
| Buy EURO Sell USD | 3/22/2004 | $ | 1.0 | 4/23/2004 | 1.2360 | $ | 1.0 | |||||
| Buy EURO Sell RUB | 3/22/2004 | $ | 3.7 | 4/23/2004 | 35.3850 | $ | 3.9 | |||||
The table below describes the forward contracts that were outstanding at December 31, 2003 and December 31, 2002:
| Foreign Currency |
Contract Date |
Forward Position |
Maturity Date |
Contract Rate |
Fair Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
(in millions) |
|
|
(in millions) |
|||||||
| At December 31, 2003 | ||||||||||||
| Buy TWD Sell Euro | 12/2/03 | $ | 2.6 | 1/5/04 | 41.1200 | $ | 2.5 | |||||
| Buy CAD Sell Euro | 12/2/03 | $ | 1.2 | 1/5/04 | 1.5682 | $ | 1.2 | |||||
| Buy DKK Sell Euro | 12/2/03 | $ | 0.8 | 1/5/04 | 7.4410 | $ | 0.8 | |||||
| Buy SEK Sell Euro | 12/2/03 | $ | 0.8 | 1/5/04 | 9.0145 | $ | 0.8 | |||||
| Buy AUD Sell Euro | 12/2/03 | $ | 1.1 | 1/5/04 | 1.6552 | $ | 1.1 | |||||
| Buy AUD Sell Euro | 12/19/03 | $ | 1.5 | 1/5/04 | 1.6810 | $ | 1.5 | |||||
Buy GBP Sell USD |
12/19/03 |
$ |
3.2 |
1/23/04 |
1.7636 |
$ |
3.2 |
|||||
| Buy SEK Sell USD | 12/19/03 | $ | 1.6 | 1/23/04 | 7.3270 | $ | 1.7 | |||||
| Buy JPY Sell USD | 12/19/03 | $ | 14.0 | 1/23/04 | 107.7050 | $ | 14.1 | |||||
| Buy EUR Sell USD | 12/19/03 | $ | 1.0 | 1/23/04 | 1.2381 | $ | 1.0 | |||||
At December 31, 2002 |
||||||||||||
| Buy USD/Sell Mexican Peso | 12/3/02 | $ | 10.6 | 1/6/03 | 10.21 | $ | 10.8 | |||||
| Buy USD/Sell Brazilian Real | 12/12/02 | $ | 1.0 | 1/16/03 | 3.74 | $ | 0.1 | |||||
All foreign subsidiaries, excluding those operating in hyper-inflationary environments, designate their local currencies as their functional currency. At March 31, 2004, the total amount of foreign subsidiary cash was $72.7 million, of which $7.7 million was invested in U.S. dollars. At December 31, 2003, the total amount of cash held by foreign subsidiaries, primarily in Japan and Korea, was $63.4 million, of which $3.7 million was invested in U.S. dollars.
61
Interest Rate Risk
We have maintained an investment portfolio of high-quality marketable securities. According to our investment policy, we may invest in taxable and tax exempt instruments including asset-backed securities. In addition, the policy establishes limits on credit quality, maturity, issuer and type of instrument. We do not use derivative instruments to hedge our investment portfolio.
The table below presents principal cash flows and interest rates by maturity dates and the fair values of our borrowings as of March 31, 2004. Fair values for fixed rate borrowings have been determined based on recent market trade values. The fair values for variable rate borrowings approximate their carrying value. Variable interest rates disclosed represent the rates on the borrowings at March 31, 2004. Interest rate risk related to the our capital leases is not significant.
| |
Expected Maturity Date |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2005 |
2006 |
2007 |
2008 |
Thereafter |
Total |
Fair Value |
|||||||||||||||||
| |
(Dollars in millions) |
||||||||||||||||||||||||
| Long-term Debt | |||||||||||||||||||||||||
| Fixed Rate | — | — | — | — | — | $ | 158.2 | $ | 158.2 | $ | 184.8 | ||||||||||||||
| Average Interest Rate | 11.75 | % | |||||||||||||||||||||||
| Variable Rate | $ | 13.1 | $ | 17.4 | $ | 17.4 | $ | 17.4 | $ | 10.2 | $ | — | $ | 75.5 | $ | 75.5 | |||||||||
| Average Interest Rate | 3.67 | % | 3.67 | % | 3.67 | % | 3.67 | % | 3.67 | % | |||||||||||||||
| Fixed Rate | — | — | — | — | — | $ | 267.5 | $ | 267.5 | $ | 286.7 | ||||||||||||||
| Average Interest Rate | 9.5 | % | |||||||||||||||||||||||
The table below presents principal cash flows and interest rates by maturity dates and the fair values of our borrowings at December 31, 2003. Fair values for fixed rate borrowings have been determined based on market trade values. The fair values for variable rate borrowings approximate their carrying value. Variable interest rates disclosed represent the rates on the borrowings at December 31, 2003. Interest rate risk related to our capital leases at the time was not significant.
| |
Expected Maturity Date |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2004 |
2005 |
2006 |
2007 |
2008 |
Thereafter |
Total |
Fair Value |
|||||||||||||||||
| |
(dollars in millions) |
||||||||||||||||||||||||
Long-term Debt |
|||||||||||||||||||||||||
| Fixed Rate | — | — | — | — | — | $ | 158.2 | $ | 158.2 | $ | 189.6 | ||||||||||||||
| Average Interest Rate | — | — | — | — | — | 11.75 | % | 11.75 | % | — | |||||||||||||||
| Variable Rate | $ | 66.1 | $ | 15.0 | $ | 15.0 | $ | 15.0 | $ | 8.7 | — | $ | 119.8 | $ | 119.8 | ||||||||||
| Average Interest Rate | 5.14 | % | 5.14 | % | 5.14 | % | 5.14 | % | 5.14 | % | — | 5.14 | % | — | |||||||||||
| Fixed Rate | — | — | — | — | — | $ | 38.0 | $ | 38.0 | $ | 38.0 | ||||||||||||||
| Average Interest Rate | — | — | — | — | — | 15.5 | % | 15.5 | % | — | |||||||||||||||
The interest rate cap is used to hedge the interest rate exposure on the term loan which has a variable interest rate. It provides protection in the event the LIBOR rates increases beyond the cap rate.
62
The table below describes the interest rate cap that was outstanding:
| Interest Rate |
Notional Amount |
Cap Rate |
Fair Value |
Maturity Date |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
|
(in millions) |
|
||||||
| At March 31, 2004 | ||||||||||
| Interest Rate Cap | $ | 32.5 | 5 | % | $ | — | 10/31/2005 | |||
At December 31, 2003 |
||||||||||
| Interest Rate Cap | $ | 34.4 | 5 | % | $ | — | 10/31/2005 | |||
At December 31, 2002 |
||||||||||
| Interest Rate Cap | $ | 43.8 | 5 | % | $ | 0.1 | 10/31/2005 | |||
Critical Accounting Policies
Our accounting policies are described in Note 2 in the Notes to Consolidated Financial Statements contained herein. Our Consolidated Financial Statements are prepared in conformity with accounting principles generally accepted in the United States, which require us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the year. Actual results could differ from those estimates. We consider the following policies to be most critical in understanding the judgments that are involved in preparing the financial statements and the uncertainties that could impact its results of operations, financial condition and cash flows.
Allowances for product returns are provided at the time the product is shipped. This accrual is based upon historic trends and experience. If the actual product returns differ from past experience, changes in the allowances are made.
We write down our inventory to provide for estimated obsolete or unsalable inventory based on assumptions about future demand for our products and market conditions. If future demand and market conditions are less favorable than management's assumptions, additional inventory write-downs could be required. Likewise, favorable future demand and market conditions could positively impact future operating results if written-off inventory is sold.
We perform goodwill impairment tests on an annual basis and on an interim basis if an event or circumstance indicates that it is more likely than not that impairment has occurred. We assess the impairment of other amortizable intangible assets and long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important that could trigger an impairment review include significant underperformance to historical or projected operating results, substantial changes in our business strategy and significant negative industry or economic trends. If such indicators are present, we evaluate the fair value of the goodwill of the reporting unit to its carrying value. For other intangible assets and long-lived assets, we determine whether the sum of the estimated undiscounted cash flows attributable to the assets in question is less than their carrying value. If it is less, we recognize an impairment loss based on the excess of the carrying amount of the assets over their respective fair values. Fair value of goodwill, other intangible assets and long-lived assets is determined by discounted future cash flows, appraisals or other methods. If the long-lived asset determined to be impaired is to be held and used, we recognize an impairment charge to the extent the present value of anticipated net cash flows attributable to the asset are less than the asset's carrying value. The fair value of the long-lived asset then becomes the asset's new carrying value, which we depreciate over the remaining estimated useful life of the asset. To the extent we determine there are indicators of impairment in future periods, write-downs may be required.
Contingencies are accounted for in accordance with SFAS 5, "Accounting for Contingencies." SFAS 5 requires that we record an estimated loss from a loss contingency when information available prior to issuance of our financial statements indicates that it is probable that an asset has been
63
impaired or a liability has been incurred at the date of the financial statements and the amount of the loss can be reasonably estimated. Accounting for contingencies such as legal and income tax matters requires us to use judgment. Many of these legal and tax contingencies can take years to be resolved. Generally, as the time period increases over which the uncertainties are resolved, the likelihood of changes to the estimate of the ultimate outcome increases. However, an adverse outcome in these matters could have a material impact on our financial condition and operating results.
Deferred income tax assets have been established for net operating loss carryforwards of certain foreign subsidiaries and have been reduced by a valuation allowance to reflect them at amounts estimated to be ultimately recognized. The net operating loss carryforwards expire in varying amounts over a future period of time. Realization of the income tax carryforwards is dependent on generating sufficient taxable income prior to expiration of the carryforwards. Although realization is not assured, we believe it is more likely than not that the net carrying value of the income tax carryforwards will be realized. The amount of the income tax carryforwards that is considered realizable, however, could change if estimates of future taxable income during the carryforward period are adjusted.
New Accounting Pronouncements
In December 2003, the SEC issued Staff Accounting Bulletin ("SAB") No. 104, "Revenue Recognition," which codifies, revises, and rescinds certain sections of SAB No. 101, "Revenue Recognition," in order to make this interpretive guidance consistent with current authoritative accounting and auditing guidance and SEC rules and regulations. The changes noted in SAB No. 104 did not have a material effect on our consolidated results of operations, consolidated financial position, or consolidated cash flows.
In May 2003, the FASB issued SFAS 150, "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity." SFAS 150 establishes standards on the classification and measurement of certain instruments with characteristics of both liabilities and equity. SFAS 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. SFAS 150 requires the classification of any financial instruments with a mandatory redemption feature, an obligation to repurchase equity shares, or a conditional obligation based on the issuance of a variable number of its equity shares, as a liability. The adoption of SFAS 150 did not have a material effect on our consolidated financial statements.
In April 2003, the FASB issued SFAS 149, "Amendment of Statement 133 on Derivative Instruments and Hedging Activities," effective for contracts entered into or modified after June 30, 2003. This amendment clarifies when a contract meets the characteristics of a derivative, clarifies when a derivative contains a financing component, and amends certain other existing pronouncements. The adoption of SFAS 149 did not have a material effect on our consolidated financial statements.
The FASB issued Interpretation 46 ("FIN 46"), "Consolidation of Variable Interest Entities" in January 2003, and a revised interpretation of FIN 46 ("FIN 46-R"). FIN 46 requires certain variable interest entities ("VIEs") to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have the characteristics of a controlling financial interest or sufficient equity to finance its activities without additional subordinated financial support from other parties. The provisions of FIN 46 are effective immediately for all arrangements entered into after January 31, 2003. We have not invested in any entities that we believe are variable interest entities for which we are the primary beneficiary. The adoption of FIN 46 and FIN 46-R had no impact on our financial position, results of operations, or cash flows.
In November 2002, the FASB issued Interpretation No. 45 ("FIN 45"), "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others," which addresses the disclosure to be made by a guarantor in its interim and annual financial
64
statements about its obligations under guarantees. The disclosure requirements are effective for interim and annual financial statements ending after December 15, 2002. We do not have any material guarantees that require disclosure under FIN 45.
FIN 45 also requires the recognition of a liability by a guarantor at the inception of certain guarantees. FIN 45 requires the guarantor to recognize a liability for the non-contingent component of a guarantee, which is the obligation to stand ready to perform in the event that specified triggering events or conditions occur. The initial measurement of this liability is the fair value of the guarantee at inception. The recognition of the liability is required even if it is not probable that payments will be required under the guarantee or if the guarantee was issued with a premium payment or as part of a transaction with multiple elements. The initial recognition and measurement provisions are effective for all guarantees within the scope of FIN 45 issued or modified after December 31, 2002. We have adopted the disclosure requirements of FIN 45 and will apply the recognition and measurement provisions for all guarantees entered into or modified after December 31, 2002.
As noted above, we have adopted the disclosure requirements of FIN 45 and will apply the recognition and measurement provisions for all guarantees entered into or modified after December 31, 2002. As of March 31, 2004, we have not entered into any guarantees within the scope of FIN 45.
65
General
We are a worldwide marketer of weight management, inner nutrition and Outer Nutrition® products that support our customers' wellness and healthy lifestyles. We market and sell these products through a global network marketing organization comprised of over one million independent distributors in 58 countries. Throughout our 24-year operating history, the high quality of our products and the effectiveness of our network marketing organization have been the primary drivers of our growth and geographic expansion. For the year ended December 31, 2003, our net sales and EBITDA were approximately $1.2 billion and $167.7 million, respectively.
Company History
Herbalife was founded in 1980 to offer safe and effective alternatives to weight management solutions then in the market. From inception, we have utilized a direct selling distribution model, as this model provides a unique and effective method for Herbalife distributors to offer personal attention to consumers of weight management products. Our distributor base grew quickly, by increasing penetration in the United States and by expanding abroad. Throughout the 1980's, we began operations in other English-speaking countries such as Canada, the United Kingdom, Australia and New Zealand, and subsequently entered several of our larger international markets including Mexico (1989), Japan (1989), Germany (1990), Italy (1992) and South Korea (1996). In conjunction with our entry into new markets, we expanded our product portfolio with new product introductions in our weight management and inner nutrition categories and introduced our Outer Nutrition® product line in 1995.
Historically, Herbalife was managed as a grass-roots enterprise with an entrepreneurial management approach. Following the death of our founder, Mark Hughes, in May 2000, the then current management team continued to run Herbalife, but began to explore strategic options including a sale of the company. As a result of this process, in July 2002, Herbalife was acquired by Whitney, Golden Gate and their affiliates.
Since the Acquisition, we have worked to strengthen our management team with experienced executives focused on making Herbalife the leading weight management and nutrition company worldwide. Since 2002, we have made several important additions to our management team that we believe will strengthen our capabilities in product development, operations and marketing. Our key hires are as follows:
66
Chairman and CEO until it was sold in 1998 to Nu Skin Enterprises, Inc., a NYSE listed company.
Our management team also draws significant support from our many veteran members, including William D. Lowe (joined in 1998), our Senior Vice President, Principal Financial and Accounting Officer, Carol Hannah (joined in 1984), our President of Distributor Communications and Support and former Co-President, and Brian L. Kane (joined in 1993), our President of Herbalife's European region and former Co-President.
We believe that the efforts of this team are already being reflected in our recent operating success and continued improvement in our financial performance. For a detailed description of our employment arrangements with our management team, see "Management—Employment Contracts."
In addition, we recently extended an offer to Richard Goudis to serve as our new Chief Financial Officer for a three-year term commencing on June 14, 2004, which Mr. Goudis accepted on June 1, 2004.
Business Strategy
Our business strategy is comprised of the following principal elements:
67
is similarly enhanced. We also believe that sales of our weight management and inner nutrition products are strengthened by the first-hand, testimonial proof of product effectiveness provided by many of our distributors, who are consumers of our products themselves.
Product Overview
For 24 years, our products have helped distributors and customers from around the world lose weight, improve their health and experience life-changing results. We have built our heritage on developing formulas that blend the best of nature with innovative techniques from nutritional science, appealing to the growing base of consumers seeking to live a healthier lifestyle.
We group our products into three categories: weight management, which consists of a full range of meal replacement programs and healthy snacks; inner nutrition, which consists of nutritional supplements; and Outer Nutrition®, which represents personal-care products. To ensure ease and simplicity of use, we have created program solutions that draw upon our experience of developing
68
results-oriented and conveniently designed products. Our program solutions target specific consumer markets such as women, men, mature adults, sports enthusiasts, as well as weight-loss and weight-management customers and individuals looking to enhance their overall well-being.
Herbalife's broad-reaching products and programs are sold through an international network of more than one million independent distributors. We support our distributors' success by offering complementary sales and marketing materials, comprehensive live training, internet-based educational programs and a growing library of audiotapes, videos, CDs and DVDs geared toward product and business-opportunity knowledge.
The following chart summarizes our net-sales information by product category during the indicated periods.
| |
Net sales by category year ended December 31, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2001 |
2002 |
2003 |
|||||||||||||
| Product category |
Net sales |
Percent of sales |
Net sales |
Percent of sales |
Net sales |
Percent of sales |
||||||||||
| |
(dollars in millions) |
|||||||||||||||
| Weight-Management | $ | 421.9 | 41.4 | % | $ | 465.6 | 42.6 | % | $ | 500.1 | 43.1 | % | ||||
| Inner Nutrition | 443.7 | 43.5 | % | 476.3 | 43.6 | % | 505.1 | 43.6 | % | |||||||
| Outer Nutrition® | 106.2 | 10.4 | % | 108.7 | 9.9 | % | 105.7 | 9.1 | % | |||||||
| Literature, Promotional and Other | 48.3 | 4.7 | % | 43.1 | 3.9 | % | 48.5 | 4.2 | % | |||||||
| Total | $ | 1,020.1 | 100.0 | % | $ | 1,093.7 | 100.0 | % | $ | 1,159.4 | 100.0 | % | ||||
Weight management
We believe that our products have helped millions of people manage their weight safely and effectively by providing our customers with numerous weight-management formulas. Among the products we offer are meal replacements, weight-loss accelerators and a variety of healthy snacks. These products include the following: (1) Formula 1 Protein Drink Mix, a meal-replacement protein powder available in five different flavors, including a kosher selection; (2) HPLC Shake Mix, a meal-replacement formula developed for consumers interested in lowering their carbohydrate intake, available in two flavors; (3) Performance Protein Powder, a high-quality soy and whey protein source developed to be added to our meal replacements to boost protein intake and decrease hunger; (4) Formula 2 Multivitamin-Mineral & Herbal Tablets, which provide essential vitamins and nutrients and are part of our weight-management programs; (5) accelerators, including Total Control™, Green Ephedra Free, and Snack Defense™, which address specific challenges associated with dieting; and (6) healthy snacks, formulated to provide between-meal nutrition and satisfaction. For the year ended December 31, 2003, $500.1 million or 43.1% of our net sales were derived from weight management products.
Inner nutrition
We market numerous dietary and nutritional supplements designed to meet our customers' specific nutritional needs. Each of these supplements contains high-quality herbs, vitamins, minerals and other natural ingredients and focuses on specific health concerns of our customers. For example, in 2003, Herbalife introduced Niteworks™, developed by Nobel Laureate in Medicine, Dr. Louis Ignarro. Niteworks™ supports energy, circulatory and vascular health and enhances blood flow to the heart, brain and other vital organs. For the year ended December 31, 2003, $505.1 million or 43.6% of our net sales were derived from inner nutrition products.
69
Outer Nutrition®
Our Outer Nutrition® products complement our weight-management and inner nutrition products and improve the appearance of our customers' body, skin and hair. These products include skin cleansers, toners, moisturizers and facial masks, shampoos and conditioners, body-wash items and a selection of fragrances for men and women. The Outer Nutrition® line is designed to make our customers look and feel their best. For the year ended December 31, 2003, $105.7 million or 9.1% of our net sales were derived from Outer Nutrition® products.
Literature, promotional and other products
We also sell literature and promotional materials, including sales aids, informational audiotapes, videotapes, CDs and DVDs, and distributor kits (called "International Business Packs"). Historically, product returns and buy-backs have not been significant. For the year ended December 31, 2003, $48.5 million or 4.2% of our net sales were derived from literature and promotional materials.
Product Manufacturing and Development
New product ideas have in the past been derived from a number of sources, including trade publications, scientific and health journals, our executives, staff and consultants and outside parties. Going forward, this process will be enhanced by our Scientific Advisory Board, which includes leading scientists, and our Medical Advisory Board, which includes leading medical doctors from around the world. In early 2003, we contributed to the establishment of the UCLA Lab at the UCLA Center for Human Nutrition. The UCLA Lab's mission is to advance nutritional science to new levels of understanding by using the most progressive research and development technologies available. The UCLA Lab will enable UCLA researchers for the first time to fingerprint herbs and to couple this with tests of the effects of herbs on living cells. We intend to take full advantage of the expertise at UCLA and other major universities by committing to support research that will utilize herbs that have been identified and profiled using these state-of-the-art techniques.
Since our products consist of herbs, vitamins, minerals and other ingredients that we regard as safe when taken as suggested by us, we do not generally conduct clinical studies of our products. However, in advance of introducing products into our markets, local counsel and other representatives, retained by us, investigate product formulation matters as they relate to regulatory compliance and other issues if necessary. Our products are then reformulated to suit both the regulatory and marketing requirements of the particular market.
All of our products are manufactured by outside companies, with the exception of products distributed in and sourced from China where we have our own manufacturing facility. All manufacturing companies are Herbalife quality and production certified. Our weight management products and nutritional supplements are manufactured for us by multiple manufacturers. We have established excellent relationships with these manufacturers and obtained improvements in supply services, product quality and product delivery. Historically, we have not been subject to material price increases by our suppliers, and we believe that in the event of price increases, we have the ability to respond to a portion of the price increases by raising the prices of our products. We own the proprietary rights to substantially all of our weight management products and dietary and nutritional supplements.
Product Return and Buy-Back Policies
In most markets, our products include a customer satisfaction guarantee. Under this guarantee, within 30 days of purchase, any customer who is not satisfied with an Herbalife product for any reason may return it or any unused portion of it to the distributor from whom it was purchased for a full refund from the distributor or credit toward the purchase of another Herbalife product. If they return
70
the products to us on a timely basis, distributors may obtain replacements from us for such returned products. In addition, in most jurisdictions, we maintain a buy-back program, pursuant to which we will repurchase products sold to a distributor provided that the distributor resigns as an Herbalife distributor, returns the product in marketable condition generally within twelve months of original purchase and meets certain documentation and other requirements. We believe this buy-back policy addresses a number of the regulatory compliance issues pertaining to network marketing systems.
Historically, product returns and buy-backs have not been significant. Product returns, refunds and buy-backs approximated 1.9%, 2.4% and 2.5% of retail sales in 2003, 2002 and 2001, respectively.
Network Marketing System
General
Our products are distributed through a global network marketing organization comprised of over one million independent distributors in 58 countries as of March 31, 2004, except in China where our sales are regulated to be conducted on a wholesale basis to local retailers.
We believe that the direct-selling distribution channel is ideally suited to selling our products, because sales of weight management and wellness products are strengthened by ongoing personal contact between retail consumers and distributors. This personal contact enhances the education of consumers in the weight management and nutrition markets and the motivation of consumers to begin and maintain weight management programs for healthy living. In addition, our distributors use Herbalife's products themselves providing first-hand testimonial proof of product effectiveness, which serves as a powerful sales tool.
We provide our distributors attractive and flexible income and career opportunities by selling our high-quality products. Our distributors have the opportunity to earn income and receive non-financial rewards designed to motivate and recognize individual achievement. As a result, we believe the income opportunity provided by our network marketing system appeals to a broad cross-section of people throughout the world, particularly those seeking to supplement family income, start a home business or pursue non-conventional, full or part-time employment opportunities. Our distributors, who are independent contractors, earn income on their own sales and can also earn royalties and bonuses on sales made by the distributors in their sales organizations. We believe our network marketing system provides an attractive income opportunity relative to other network marketing companies.
In connection with the Acquisition, we entered into an agreement with our distributors that no material changes adverse to the distributors will be made to the existing marketing plan and that we will continue to distribute our products exclusively through our independent distributors.
Structure of the network marketing system
To become a distributor, a person must be sponsored by an existing distributor, except in China where no sponsorship is allowed, and must purchase an International Business Pack from us, except in South Korea, where there is no charge for a distributor kit. To become a supervisor or qualify for a higher level, distributors must achieve specified volumes of product purchases or earn certain amounts of royalty overrides during specified time periods and must re-qualify for the levels once each year. To attain supervisor status, a distributor generally must purchase products representing at least 4,000 volume points in one month or 2,500 volume points in two consecutive months. China has its own unique qualifying program. Volume points are point values assigned to each of our products that are equal in all countries and are based on the suggested retail price of U.S. products. Supervisors may then attain higher levels, which consist of the Global Expansion Team, the Millionaire Team and the President's Team, by earning increasing amounts of royalty overrides based on purchases by distributors within their organizations. Supervisors contribute significantly to our sales and some key supervisors
71
who have attained the highest levels within our distributor network are responsible for their organization's generation of a substantial portion of our sales and for recruiting a substantial number of our distributors.
The following table sets forth the approximate number of our supervisors at the dates indicated:
| |
February* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2000 |
2001 |
2002 |
2003 |
2004** |
|||||
| Approximate number of supervisors | 160,000 | 165,000 | 172,000 | 173,000 | 191,000 | |||||
Distributor earnings
Distributor earnings are derived from several sources. First, distributors may earn profits by purchasing our products at wholesale prices, which are discounted 25% to 50% from suggested retail prices, depending on the distributors' level within our distributor network, and selling our products to retail customers or to other distributors. Second, distributors who sponsor other distributors and establish their own sales organizations may earn (i) royalty overrides, 15% of product retail sales in the aggregate, (ii) production bonuses, 7% of product retail sales in the aggregate and (iii) President's Team bonus, 1% of product retail sales in the aggregate. In China, distributors are limited to earn profits from retailing our products by purchasing our products with discounts and rebates up to 50% of suggested retail price and then reselling them to customers. Distributors may also earn a 5% or 10% sales volume bonus on their own purchases.
Distributors earn the right to receive royalty overrides upon attaining the level of supervisor and above, and production bonuses upon attaining the level of Global Expansion Team and above. Once a distributor becomes a supervisor, he or she has an incentive to qualify, by earning specified amounts of royalty overrides, as a member of the Global Expansion Team, the Millionaire Team or the President's Team, and thereby receive production bonuses of up to 7%. We believe that the right of distributors to earn royalty overrides and production bonuses contributes significantly to our ability to retain our most productive distributors.
We seek to expand our distributor base in each market by offering distributors attractive compensation opportunities. We believe our international sponsorship program provides a significant advantage to our distributors as compared with distributors in some other network marketing organizations. This program permits distributors to sponsor distributors in other countries where we are licensed to do business and where we have obtained required product approvals.
Distributor training
We and our distributor leadership conduct over 25,000 training sessions annually on local, regional and global levels to educate and motivate our distributors. In addition to these training sessions, we have our own "Herbalife Broadcast Network" that we use to provide distributors continual training and information. The Herbalife Broadcast Network can be seen on the DISH Network satellite television
72
service and on the internet. We use the Herbalife Broadcast Network to provide our distributors with the most current product and marketing information.
Geographic Presence
The following chart sets forth the markets in which we currently operate, the number of countries open in such markets as of March 31, 2004 and net sales information by region during the past three fiscal years.
| |
Year ended December 31, |
Percent of total net sales 2003 |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geographic region |
Number of countries open as of March 31, 2004 |
||||||||||||
| 2001 |
2002 |
2003 |
|||||||||||
| |
(dollars in millions) |
||||||||||||
| Americas | $ | 386.9 | $ | 424.3 | $ | 424.4 | 36.6 | % | 11 | ||||
| Europe | 283.2 | 342.7 | 448.2 | 38.7 | % | 34 | |||||||
| Asia/Pacific Rim | 172.0 | 185.5 | 167.5 | 14.4 | % | 12 | |||||||
| Japan | 178.0 | 141.2 | 119.3 | 10.3 | % | 1 | |||||||
| Total | $ | 1,020.1 | $ | 1,093.7 | $ | 1,159.4 | 100.0 | % | 58 | ||||
We conduct business in 58 countries located in The Americas, Europe, Asia/Pacific Rim and Japan. Net sales in those regions represented 36.6%, 38.7%, 14.4% and 10.3%, respectively, of our total net sales in 2003. The fiscal year ended December 31, 2003 marks the first year in which we separately recognize revenues from sales to distributors in Japan and the net sales information reported in the table above for prior periods reflects the net sales attributable to that market during those periods. For more information about our results of operation in these four geographic regions, see Note 11 in the Notes to Consolidated Financial Statements included elsewhere herein.
Historically a significant portion of our sales have been from a few countries. In 2003, our top six countries accounted for approximately 56.4% of total net sales. Over the most recent five years, the top six countries of each year have gone from representing approximately 72.5% of net sales in 1999 to 56.4% of net sales in 2003.
After entering a new country, we have in many instances experienced an initial period of rapid growth in sales as new distributors were recruited, followed by a decline in sales. We believe that a significant factor affecting these markets has been the opening of other new markets within the same geographic region or with the same or similar language or cultural bases and the corresponding tendency of some distributors to focus their attention on the business opportunities provided by new markets instead of developing their established sales organizations in existing markets. Additionally, in some instances, we have become aware that certain sales in certain existing markets were attributable to purchasers who distributed our products in countries that had not yet been opened. When these countries were opened, the sales in existing markets shifted to the newly opened markets, resulting in a decline in sales in the existing markets. To the extent we determine to open new markets in the future, we will continue to seek to minimize the impact on distributor focus in existing markets and to ensure that adequate distributor support services and other Herbalife systems are in place to support growth.
A significant factor contributing to potential sales declines is adverse publicity. We believe that unfavorable press reports are generally based upon a lack of familiarity with us and our network marketing system and often originate from competitive forces in the local market. The effect of occasional adverse publicity has at times also led to increased regulatory scrutiny in some countries, which may also have an adverse effect on sales.
Our business strategy includes placing greater emphasis on sustaining and increasing sales levels in our existing markets, particularly where we have experienced declines in sales after an initial period of
73
growth. We seek greater market penetration in our markets by refining sales, marketing and recruiting initiatives and by increasing the availability of our existing products in those markets where we currently offer only a small percentage of our full product line. See "—Business Strategy." A foreign government may impose trade or foreign exchange restrictions or increased tariffs, which could adversely affect our operations. We are also exposed to risks associated with foreign currency fluctuations. For instance, purchases from suppliers are generally made in U.S. dollars while sales to distributors are generally made in local currencies. Accordingly, strengthening of the U.S. dollar versus a foreign currency could have a negative impact on us. Although we engage in transactions to protect against risks associated with foreign currency fluctuations, we cannot be certain any hedging activity will effectively reduce our exchange rate exposure. Our operations in some markets also may be adversely affected by political, economic and social instability in foreign countries. As we continue to focus on expanding our existing international operations, these and other risks associated with international operations may increase. Approximately 76% of our net sales for the year ended December 31, 2003 were generated outside the United States.
Operations
Our global distribution system features centralized distribution and telephone ordering systems coupled with storefront distributor service centers. Distribution and service centers are conveniently located and attractively designed in order to encourage local distributors to meet and network with each other and learn more about our products, marketing system and upcoming events. In addition, they can showcase the business while improving their selling productivity. Our major distribution warehouses have been automated with "pick-to-light" picking systems which consistently deliver over 99.5% order accuracy and handling systems that provide for inspection of every shipment before it is sent to delivery. Shipping and processing standards for orders placed are either the same day or the following business day. We have central sales ordering facilities for answering and processing telephone orders. Operators at such centers are capable of conversing in multiple languages.
Our weight management, inner nutrition and Outer Nutrition® products are distributed to foreign markets either from the facilities of our manufacturers or from our Los Angeles and Venray, Netherlands distribution centers. Products are distributed in the United States market from our Los Angeles distribution center or from our Memphis distribution center. Nutrition products manufactured in countries globally are generally transported by truck, cargo ship or plane to our international markets and are warehoused in either one of our foreign distribution centers or a contracted third party warehouse and distribution center. After arrival of the products in a foreign market, distributors purchase the products from the local distribution center or the associated sales center. Our Outer Nutrition® products are predominantly manufactured in Europe and the United States. The products manufactured in Europe are shipped to a centralized warehouse facility, from which delivery by truck, ship or plane to other international markets occurs.
Management Information, Internet and Telecommunication Systems
In order to facilitate our continued growth and support distributor activities, we continually upgrade our management information, internet and telecommunication systems. These systems include: (i) a centralized host computer located in Southern California, which is linked to our international markets through a dedicated wide area network that provides on-line, real-time computer connectivity and access; (ii) local area networks of personal computers within our markets, serving our regional administrative staffs; (iii) an international e-mail system through which our employees communicate; (iv) a standardized Northern Telecom Meridian telecommunication system in most of our markets; (v) fully integrated Oracle supply chain management system that has been installed in our distribution centers; and (vi) internet websites to provide a variety of online services for distributors (status of qualifications, meeting announcements, product information, application forms, educational materials and, in the United States, sales ordering capabilities). These systems are designed to provide financial
74
and operating data for management, timely and accurate product ordering, royalty override payment processing, inventory management and detailed distributor records. We intend to continue to invest in our systems in order to strengthen our operating platform.
Regulation
General. In both our United States and foreign markets, we are affected by extensive laws, governmental regulations, administrative determinations, court decisions and similar constraints. Such laws, regulations and other constraints may exist at the federal, state or local levels in the United States and at all levels of government in foreign jurisdictions, including regulations pertaining to: (i) the formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of our products; (ii) product claims and advertising, including direct claims and advertising by us, as well as claims and advertising by distributors, for which we may be held responsible; (iii) our network marketing system; (iv) transfer pricing and similar regulations that affect the level of U.S. and foreign taxable income and customs duties; and (v) taxation of distributors (which in some instances may impose an obligation on us to collect the taxes and maintain appropriate records).
Products. The formulation, manufacturing, packaging, storing, labeling, promotion, advertising, distribution and sale of our products are subject to regulation by various governmental agencies, including (i) the Food and Drug Administration ("the FDA"), (ii) the Federal Trade Commission (the "FTC"), (iii) the Consumer Product Safety Commission ("CPSC"), (iv) the United States Department of Agriculture ("USDA"), (v) the Environmental Protection Agency ("EPA"), and (vi) the United States Postal Service. Our activities also are regulated by various agencies of the states, localities and foreign countries in which our products are manufactured, distributed and sold. The FDA, in particular, regulates the formulation, manufacture and labeling of foods, dietary supplements, cosmetics and over-the-counter ("OTC") drugs, such as those distributed by us. FDA regulations require us and our suppliers to meet relevant current good manufacturing practice ("CGMP") regulations for the preparation, packing and storage of foods and OTC drugs. On March 7, 2003, the FDA released for comment its proposed CGMP's for dietary supplements.
Most OTC drugs are subject to FDA Monographs that establish labeling and composition for these products. Our products must comply with these Monographs, and our manufacturers must list all products with the FDA and follow CGMP. Our cosmetic products are regulated for safety by the FDA, which requires that ingredients meet industry standards for non-allergenicity and non-toxicity. Performance claims for cosmetics may not be "therapeutic."
The 1994 Dietary Supplement Health and Education Act ("DSHEA") revised the provisions of the Federal Food, Drug and Cosmetic Act ("FFDCA") concerning the composition and labeling of dietary supplements and, we believe, is generally favorable to the dietary supplement industry. The legislation creates a new statutory class of dietary supplements. This new class includes vitamins, minerals, herbs, amino acids and other dietary substances for human use to supplement the diet, and the legislation grandfathers, with some limitations, dietary ingredients that were on the market before October 15, 1994. A dietary supplement that contains a dietary ingredient that was not on the market before October 15, 1994 will require evidence of a history of use or other evidence of safety establishing that it is reasonably expected to be safe. Manufacturers of dietary supplements that make specified types of statements on dietary supplements, including some product performance claims, must have substantiation that the statements are truthful and not misleading. The majority of the products marketed by us are classified as dietary supplements under the FFDCA.
In January 2000, the FDA published a final rule that defines the types of statements that can be made concerning the effect of a dietary supplement on the structure or function of the body pursuant to DSHEA. Under DSHEA, dietary supplement labeling may bear structure or function claims, which are claims that the products affect the structure or function of the body, without prior FDA approval. They may not bear a claim that they can prevent, treat, cure, mitigate or diagnose disease (a disease
75
claim). The new final rule describes how the FDA will distinguish disease claims from structure or function claims. In February 2001, the FDA issued a notice requesting comments on the types of information that should be included in guidance to applying the regulations on statements made for dietary supplements concerning the effect of a dietary supplement on the structure or function of the body. In January 2002, the FDA issued a Structure/Function Claims Small Entity Compliance Guide, which provided guidance to industry on the requirements that apply to determining whether a claim for a dietary supplement is a structure/function claim or a disease claim. The Company has continued its efforts to make sure that its dietary supplement product labeling complies with the requirements of the structure or function final rule, which became effective in 2000, as well as with the more recently issued Compliance Guide.
As a marketer of dietary and nutritional supplements and other products that are ingested by consumers, we are subject to the risk that one or more of the ingredients in our products may become the subject of adverse regulatory action. A number of states restricted the sale of dietary supplements containing botanical sources of ephedrine alkaloids. As a result of these state regulations, Herbalife stopped sales of its dietary supplements containing botanical sources of ephedrine alkaloids due to a shift in consumer preference for "ephedra free products" and a significant increase in products liability insurance premiums for products containing botanical sources of ephedrine group alkaloids.
In addition, we have ceased sales of Thermojetics® original green herbal tablets containing ephedrine alkaloids derived from Chinese Ma Huang, as well as Thermojetics® green herbal tablets and Thermojetics® gold herbal tablets (the latter two containing the herb Sida cordifolia which is another botanical source of ephedrine alkaloids).
On February 6, 2004, the FDA published a rule finding that dietary supplements containing ephedrine alkaloids present an unreasonable risk of illness or injury under conditions of use recommended or suggested in the labeling of the product, or, if no conditions of use are suggested in the labeling, under ordinary conditions of use, and are therefore adulterated under Section 402(f)(1)(A) of the FFDCA. This rule will become effective 60 days after publication so as to allow for congressional review in accordance with 5 U.S.C. 801-808. As noted earlier, we decided to cease marketing dietary supplements containing ephedra well before the FDA's action. As of January 2003, we stopped selling products containing ephedrine alkaloids.
The FDA has on record a small number of reports of adverse reactions allegedly resulting from the ingestion of our Thermojetics® original green tablet. Many other companies manufacture products containing various amounts of Ma Huang, and the FDA has on record hundreds of reports of adverse reactions to these products. On September 16, 2002, the FDA changed its policies for notifying companies of anecdotal adverse event reports for dietary supplements. Since then, we have received three special nutritional adverse events reports from the FDA which were not specific to any product.
As a further outgrowth of the ephedra review, the FDA, in January 2004, announced that it would undertake a review of the safety of citrus aurantium, the ingredient we have utilized in place of ephedra. Unconfirmed reports of adverse events were disclosed by the FDA in 2004. The FDA's review has led us to consider another substitute ingredient.
The FDA's decision to ban ephedra has triggered a significant reaction by the national media, which is calling for the repeal or amendment of DSHEA. These media view supposed "weaknesses" within DSHEA as the underlying reason why ephedra was allowed to remain on the market. We have been advised that DSHEA opponents in Congress may use this anti-DSHEA momentum to advance existing or new legislation to amend or repeal DSHEA. We should expect to see the following:
76
On March 7, 2003, the FDA proposed a new regulation to require current good manufacturing practices affecting the manufacture, packing, and holding of dietary supplements. The proposed rule would establish standards to ensure that dietary supplements and dietary ingredients are not adulterated with contaminants or impurities, and are labeled to accurately reflect the active ingredients and other ingredients in the products. It also includes proposed requirements for designing and constructing physical plans, establishing quality control procedures, and testing manufactured dietary ingredients and dietary supplements, as well as proposed requirements for maintaining records and for handling consumer complaints related to CGMPs. We are evaluating this proposal with respect to its potential impact upon the various contract manufacturers that we use to manufacturer our products, some of whom might not meet the new standards. It is important to note that the proposed rule, in an effort to limit disruption, includes a three-year phase-in for small businesses of any final rule that is issued. This will mean that some of our contract manufacturers will not be fully impacted by the proposed rule until at least 2007. However, the proposed rule can be expected to result in additional testing costs.
In December 1999, we introduced a new line of weight management products that are suitable for diets that are high in protein and low in carbohydrates. The line, which consists of eight nutritionally balanced high-protein products that are also low in carbohydrates, is called the Thermojetics® HPLC Program. The FDA has not authorized the use of a low carbohydrate claim on the label of individual food products, and therefore we have not made such a claim on the label of any of the eight products that together comprise our Thermojetics® HPLC Program. We believe, however, that it is permissible to accurately describe the entire program as one that is suitable for a diet that is high in protein and low in carbohydrates, and we have elected to do so by virtue of the name that we have selected for this weight management program. In addition, we are exploring other alternatives which do not involve specifically describing the program as low in carbohydrates.
Some of the products marketed by us are considered conventional foods and are currently labeled as such. Within the United States, both this category of products and dietary supplements are subject to the Nutrition, Labeling and Education Act ("NLEA"), and regulations promulgated under the NLEA. The NLEA regulates health claims, ingredient labeling and nutrient content claims characterizing the level of a nutrient in the product. The ingredients added to conventional foods must either be generally recognized as safe by experts ("GRAS") or approved food additives under FDA regulations.
In foreign markets, prior to commencing operations and prior to making or permitting sales of our products in the market, we may be required to obtain an approval, license or certification from the country's ministry of health or comparable agency. Where a formal approval, license or certification is not required, we nonetheless seek a favorable opinion of counsel regarding our compliance with applicable laws. Prior to entering a new market in which a formal approval, license or certificate is required, we work extensively with local authorities in order to obtain the requisite approvals. The approval process generally requires us to present each product and product ingredient to appropriate regulators and, in some instances, arrange for testing of products by local technicians for ingredient analysis. The approvals may be conditioned on reformulation of our products or may be unavailable with respect to some products or some ingredients. Product reformulation or the inability to introduce some products or ingredients into a particular market may have an adverse effect on sales. We must also comply with product labeling and packaging regulations that vary from country to country. Our
77
failure to comply with these regulations can result in a product being removed from sale in a particular market, either temporarily or permanently.
The FTC, which exercises jurisdiction over the advertising of all of our products, has in the past several years instituted enforcement actions against several dietary supplement companies and against manufacturers of weight loss products generally for false and misleading advertising of some of their products. These enforcement actions have often resulted in consent decrees and monetary payments by the companies involved. In addition, the FTC has increased its scrutiny of the use of testimonials, which we also utilize. Although we have not been the target of FTC enforcement action for the advertising of our products, we cannot be sure that the FTC, or comparable foreign agencies, will not question our advertising or other operations in the future. In November 1998, the FTC issued a guide for the dietary supplement industry, describing how the FTC applies the law that it administers to advertisements for dietary supplements. It is unclear whether the FTC will subject advertisements of this kind, including our advertisements, to increased surveillance to ensure compliance with the principles set forth in the guide.
In some countries, regulations applicable to the activities of our distributors also may affect our business because in some countries we are, or regulators may assert that we are, responsible for our distributors' conduct. In these countries, regulators may request or require that we take steps to ensure that our distributors comply with local regulations. The types of regulated conduct include: (i) representations concerning our products; (ii) income representations made by us and/or distributors; (iii) public media advertisements, which in foreign markets may require prior approval by regulators; and (iv) sales of products in markets in which the products have not been approved, licensed or certified for sale.
In some markets, it is possible that improper product claims by distributors could result in our products being reviewed by regulatory authorities and, as a result, being classified or placed into another category as to which stricter regulations are applicable. In addition, we might be required to make labeling changes.
We are unable to predict the nature of any future laws, regulations, interpretations or applications, nor can we predict what effect additional governmental regulations or administrative orders, when and if promulgated, would have on our business in the future. They could, however, require: (i) the reformulation of some products not capable of being reformulated; (ii) imposition of additional record keeping requirements; (iii) expanded documentation of the properties of some products; (iv) expanded or different labeling; (v) additional scientific substantiation regarding product ingredients, safety or usefulness; and/or (vi) additional distributor compliance surveillance and enforcement action by Herbalife.
Any or all of these requirements could have a material adverse effect on our results of operations and financial condition. All of our officers and directors are subject to a permanent injunction issued in October 1986 pursuant to the settlement of an action instituted by the California Attorney General, the State Health Director and the Santa Cruz County District Attorney. We consented to the entry of this injunction without in any way admitting the allegations of the complaint. The injunction prevents us and our officers and directors from making specified claims in future advertising of our products and requires us to implement some documentation systems with respect to payments to our distributors. At the same time, the injunction does not prevent us from continuing to make specified claims concerning our products that have been made and are being made, provided that we have a reasonable basis for making the claims.
We are aware that, in some of our international markets, there has been recent adverse publicity concerning products that contain ingredients that have been genetically modified ("GM"). In some markets, the possibility of health risks or perceived consumer preference thought to be associated with GM ingredients has prompted proposed or actual governmental regulation. Some of our products
78
contain ingredients that would be or might be classified as having been genetically modified. We cannot anticipate the extent to which regulations in our markets will restrict the use of GM ingredients in our products or the impact of any regulations on our business in those markets. In response to any applicable regulations, we would, where practicable, reformulate or re-label our products to satisfy the regulations. We believe, based upon currently available information, that compliance with regulatory requirements in this area should not have a material adverse effect on us or our business. However, because publicity and governmental scrutiny of GM ingredients is a relatively new and evolving area, there can be no assurance in this regard. If a significant number of our products were found to be genetically modified and regulations in our markets significantly restricted the use of GM ingredients in our products, our business could be materially adversely affected.
In addition, in certain of our markets there has been recent adverse publicity concerning infection of bovine products and by-products by BSE, which may cause what is commonly referred to as mad cow disease. Certain of our products contain ingredients derived from bovine sources. We are not aware of any infection or contamination of any of our products by BSE. Should any such infection or contamination be detected, it could have a material adverse effect on our business. Additionally, if governments preclude importation of products from the U.S. containing bovine-derived ingredients, it could adversely impact product availability and/or future price. Further, even if no such infection or contamination is detected, adverse publicity concerning the BSE risk, or governmental or regulatory developments aimed at combating the risk of BSE contamination by regulating bovine products and/or by-products, could have a material adverse effect on our business. We anticipate some impact associated with the discovery of BSE in the United States, but do not expect this issue will have a material effect on our business.
Network marketing system. Our network marketing system is subject to a number of federal and state regulations administered by the FTC and various state agencies as well as regulations in foreign markets administered by foreign agencies. Regulations applicable to network marketing organizations generally are directed at ensuring that product sales ultimately are made to consumers and that advancement within our organization is based on sales of the organization's products rather than investments in the organizations or other non-retail sales related criteria. For instance, in some markets, there are limits on the extent to which distributors may earn royalty overrides on sales generated by distributors that were not directly sponsored by the distributor. When required by law, we obtain regulatory approval of our network marketing system or, when this approval is not required, the favorable opinion of local counsel as to regulatory compliance. Nevertheless, we remain subject to the risk that, in one or more markets, our marketing system could be found not to be in compliance with applicable regulations. Failure by us to comply with these regulations could have a material adverse effect on our business in a particular market or in general.
We also are subject to the risk of private party challenges to the legality of our network marketing system. For example, in Webster v. Omnitrition International, Inc., 79 F.3d 776 (9th Cir. 1996), the multi-level marketing program of Omnitrition International, Inc. ("Omnitrition") was successfully challenged in a class action by Omnitrition distributors who alleged that Omnitrition was operating an illegal "pyramid scheme" in violation of federal and state laws. We believe that our network marketing system satisfies the standards set forth in the Omnitrition case and other applicable statutes and case law defining a legal marketing system, in part based upon significant differences between our marketing system and that described in the Omnitrition case.
Herbalife is a defendant in a purported class action lawsuit pending in the U.S. District Court of California (Jacobs v. Herbalife International, Inc., et al) originally filed on February 19, 2002 challenging marketing practices of several distributors and Herbalife under various state and federal laws. As a result of recent rulings of the Court, only claims under federal securities law remain. We understand that the plaintiffs have refiled certain state law claims in the Superior Court of the State of California, County of San Francisco. As of the date of this prospectus, we have not been served with a complaint.
79
The parties are in discussions regarding a possible settlement but no binding settlement agreement has yet been reached. We believe that we have meritorious defenses to the suit.
Herbalife and certain of its distributors have been named as defendants in a purported class action lawsuit filed July 16, 2003 in the Circuit Court of Ohio County in the State of West Virginia (Mey v. Herbalife International, Inc., et al). Herbalife removed the lawsuit to federal court and the plaintiff sought to remand the lawsuit to state court. The plaintiff's motion was denied. The complaint alleges that certain telemarketing practices of certain Herbalife distributors violate the Telephone Consumer Protection Act and seeks to hold Herbalife liable for the practices of its distributors. We believe that we have meritorious defenses to the suit.
It is an ongoing part of our business to monitor and respond to regulatory and legal developments, including those that may affect our network marketing system. However, the regulatory requirements concerning network marketing systems do not include bright line rules and are inherently fact-based. An adverse judicial determination with respect to our network marketing system could have a material adverse effect on our business. An adverse determination could: (i) require us to make modifications to our network marketing system, (ii) result in negative publicity or (iii) have a negative impact on distributor morale. In addition, adverse rulings by courts in any proceedings challenging the legality of multi-level marketing systems, even in those not involving us directly, could have a material adverse effect on our operations.
Transfer pricing and similar regulations. In many countries, including the United States, we are subject to transfer pricing and other tax regulations designed to ensure that appropriate levels of income are reported as earned by our U.S. or local entities and are taxed accordingly. In addition, our operations are subject to regulations designed to ensure that appropriate levels of customs duties are assessed on the importation of our products.
Although we believe that we are in substantial compliance with all applicable regulations and restrictions, we are subject to the risk that governmental authorities could audit our transfer pricing and related practices and assert that additional taxes are owed. For example, we are currently subject to pending or proposed audits that are at various levels of review, assessment or appeal in a number of jurisdictions involving transfer pricing issues, income taxes, duties, value added taxes, withholding taxes and related interest and penalties in material amounts. In some circumstances, additional taxes, interest and penalties have been assessed, and we will be required to litigate to reverse the assessments. We and our tax advisors believe that there are substantial defenses to the allegations that additional taxes are owing, and we are vigorously against the imposition of additional proposed taxes. The ultimate resolution of these matters may take several years, and the outcome is uncertain.
In the event that the audits or assessments are concluded adversely to us, we may or may not be able to offset or mitigate the consolidated effect of foreign income tax assessments through the use of U.S. foreign tax credits. Currently, we anticipate utilizing the majority of our foreign tax credits in the year in which they arise with the unused amount carried forward. Because the laws and regulations governing U.S. foreign tax credits are complex and subject to periodic legislative amendment, we cannot be sure that we would in fact be able to take advantage of any foreign tax credits in the future. As a result, adverse outcomes in these matters could have a material impact on our financial condition and operating results.
Other regulations. We also are subject to a variety of other regulations in various foreign markets, including regulations pertaining to social security assessments, employment and severance pay requirements, import/export regulations and antitrust issues. As an example, in many markets, we are substantially restricted in the amount and types of rules and termination criteria that we can impose on distributors without having to pay social security assessments on behalf of the distributors and without incurring severance obligations to terminated distributors. In some countries, we may be subject to these obligations in any event.
80
Our failure to comply with these regulations could have a material adverse effect on our business in a particular market or in general. Assertions that we failed to comply with regulations or the effect of adverse regulations in one market could adversely affect us in other markets as well by causing increased regulatory scrutiny in those other markets or as a result of the negative publicity generated in those other markets.
Compliance procedures. As indicated above, Herbalife, our products and our network marketing system are subject, both directly and indirectly through distributors' conduct, to numerous federal, state and local regulations, both in the United States and foreign markets. Beginning in 1985, we began to institute formal regulatory compliance measures by developing a system to identify specific complaints against distributors and to remedy any violations by distributors through appropriate sanctions, including warnings, suspensions and, when necessary, terminations. In our manuals, seminars and other training programs and materials, we emphasize that distributors are prohibited from making therapeutic claims for our products.
Our general policy regarding acceptance of distributor applications from individuals who do not reside in one of our markets is to refuse to accept the individual's distributor application. From time to time, exceptions to the policy are made on a country-by-country basis.
In order to comply with regulations that apply to both us and our distributors, we conduct considerable research into the applicable regulatory framework prior to entering any new market to identify all necessary licenses and approvals and applicable limitations on our operations in that market. Typically, we conduct this research with the assistance of local legal counsel and other representatives. We devote substantial resources to obtaining the necessary licenses and approvals and bringing our operations into compliance with the applicable limitations. We also research laws applicable to distributor operations and revise or alter our distributor manuals and other training materials and programs to provide distributors with guidelines for operating a business, marketing and distributing our products and similar matters, as required by applicable regulations in each market. We, however, are unable to monitor our supervisors and distributors effectively to ensure that they refrain from distributing our products in countries where we have not commenced operations, and we do not devote significant resources to this type of monitoring.
In addition, regulations in existing and new markets often are ambiguous and subject to considerable interpretive and enforcement discretion by the responsible regulators. Moreover, even when we believe that we and our distributors are initially in compliance with all applicable regulations, new regulations regularly are being added and the interpretation of existing regulations is subject to change. Further, the content and impact of regulations to which we are subject may be influenced by public attention directed at us, our products or our network marketing system, so that extensive adverse publicity about us, our products or our network marketing system may result in increased regulatory scrutiny.
It is an ongoing part of our business to anticipate and respond to new and changing regulations and to make corresponding changes in our operations to the extent practicable. Although we devote considerable resources to maintaining our compliance with regulatory constraints in each of our markets, we cannot be sure that (i) we would be found to be in full compliance with applicable regulations in all of our markets at any given time or (ii) the regulatory authorities in one or more markets will not assert, either retroactively or prospectively or both, that our operations are not in full compliance. These assertions or the effect of adverse regulations in one market could negatively affect us in other markets as well by causing increased regulatory scrutiny in those other markets or as a result of the negative publicity generated in those other markets. These assertions could have a material adverse effect on us in a particular market or in general. Furthermore, depending upon the severity of regulatory changes in a particular market and the changes in our operations that would be necessitated to maintain compliance, these changes could result in our experiencing a material reduction in sales in the market or determining to exit the market altogether. In this event, we would
81
attempt to devote the resources previously devoted to the market to a new market or markets or other existing markets. However, we cannot be sure that this transition would not have an adverse effect on our business and results of operations either in the short or long-term.
Trademarks
We use the umbrella trademarks Herbalife, Thermojetics, Dermajetics, and have several other trademarks and trade names registered in connection with our products and operations. Our trademark registrations are issued through the United States Patent and Trademark Office and in comparable agencies in the foreign countries. We consider our trademarks and trade names to be an important factor in our business. We also take care in protecting the intellectual property rights of our proprietary formulas.
Competition
The business of marketing weight management, inner nutrition and Outer Nutrition® products is highly competitive. This market segment includes numerous manufacturers, distributors, marketers, retailers and physicians that actively compete for the business of consumers both in the United States and abroad. The market is highly sensitive to the introduction of new products or weight management plans, including various prescription drugs that may rapidly capture a significant share of the market. As a result, our ability to remain competitive depends in part upon the successful introduction of new products. In addition, we anticipate that we will be subject to increasing competition in the future from sellers that utilize electronic commerce. We cannot be sure of the impact of electronic commerce or that it will not adversely affect our business.
We are subject to significant competition for the recruitment of distributors from other network marketing organizations, including those that market weight management products, nutritional supplements, and personal care products, as well as other types of products. Some of our competitors are substantially larger than we are, and have available considerably greater financial resources than we have. Our ability to remain competitive depends, in significant part, on our success in recruiting and retaining distributors through an attractive compensation plan and other incentives. We believe that our production bonus program, international sponsorship program and other compensation and incentive programs provide our distributors with significant earning potential. However, we cannot be sure that our programs for recruitment and retention of distributors will be successful.
Employees
As of March 31, 2004, we had 2,247 full-time employees. These numbers do not include our distributors, who generally are independent contractors rather than our employees. Except for some employees in Mexico and in some European countries, none of our employees are members of any labor union, and we have never experienced any business interruption as a result of any labor disputes.
Properties
We lease all of our physical properties located in the United States. Our executive offices, located in Century City, California, include approximately 115,000 square feet of general office space under lease arrangements expiring in February 2006. We lease an aggregate of approximately 144,000 square feet of office space, computer facilities and conference rooms at the Operations Center in Inglewood, California, under a lease that expires in October 2006, and approximately 150,000 square feet of warehouse space in two separate facilities located in Los Angeles and Memphis. The Los Angeles and Memphis lease agreements have terms through June 2006 and August 2006, respectively. In Venray, Netherlands, we lease our European centralized warehouse of approximately 175,000 square feet. The lease expires in June 2007. We also lease warehouse, manufacturing plant and office space in a majority
82
of our other geographic areas of operation. We believe that our existing facilities are adequate to meet our current requirements and that comparable space is readily available at each of these locations.
Legal Proceedings
We are from time to time engaged in routine litigation. We regularly review all pending litigation matters in which we are involved and establish reserves deemed appropriate by management for these litigation matters when a probable loss estimate can be made.
Herbalife is a defendant in a purported class action lawsuit pending in the U.S. District Court of California (Jacobs v. Herbalife International, Inc., et al) originally filed on February 19, 2002 challenging marketing practices of several distributors and Herbalife under various state and federal laws. As a result of recent rulings of the Court, only claims under federal securities law remain. We understand that the plaintiffs have refiled certain state law claims in the Superior Court of the State of California, County of San Francisco. As of the date of this prospectus, we have not been served with a complaint. The parties are in discussions regarding a possible settlement but no binding settlement agreement has yet been reached. We believe that we have meritorious defenses to the suit.
Herbalife and certain of its distributors have been named as defendants in a purported class action lawsuit filed July 16, 2003 in the Circuit Court of Ohio County in the State of West Virginia (Mey v. Herbalife International, Inc., et al). Herbalife removed the lawsuit to federal court and the plaintiff sought to remand the lawsuit to state court. The plaintiff's motion was denied. The complaint alleges that certain telemarketing practices of certain Herbalife distributors violate the Telephone Consumer Protection Act and seeks to hold Herbalife liable for the practices of its distributors. We believe that we have meritorious defenses to the suit. At the current time, we cannot make an estimate of the range of exposure of this matter with any degree of certainty.
As a marketer of dietary and nutritional supplements and other products that are ingested by consumers or applied to their bodies, we have been and are currently subjected to various product liability claims. The effects of these claims to date have not been material to us, and the reasonably possible range of exposure on currently existing claims is not material to us. We believe that we have meritorious defenses to the allegations contained in the lawsuits. We currently maintain product liability insurance with an annual deductible of $10 million.
Certain of our subsidiaries have been subject to tax audits by governmental authorities in their respective countries. In certain of these tax audits, governmental authorities are proposing that significant amounts of additional taxes and related interest and penalties are due. We and our tax advisors believe that there are substantial defenses to their allegations that additional taxes are owing, and we are vigorously contesting the additional proposed taxes and related charges. These matters may take several years to resolve, and we cannot be sure of their ultimate resolution. However, it is the opinion of management that adverse outcomes, if any, will not likely result in a material effect on our financial condition and operating results.
83
Biographical information follows for each person who serves as a director and/or an executive officer of Holdings and Herbalife. The table sets forth certain information regarding these individuals (ages are as of June 9, 2004).
| Name** |
Age |
Position with Herbalife |
Director/Officer Since* |
|||
|---|---|---|---|---|---|---|
| Peter M. Castleman(4)(5) | 48 | Chairman of the Board | 2002 | |||
Michael O. Johnson(3)(6) |
49 |
Chief Executive Officer, Director |
2003 |
|||
Gregory Probert(6) |
47 |
Chief Operating Officer |
2003 |
|||
Henry Burdick(4)(6) |
62 |
Vice Chairman, Director |
2002 |
|||
William D. Lowe |
54 |
Senior Vice President, Principal Financial and Accounting Officer |
1998 |
|||
Matthew Wisk |
44 |
Chief Marketing Officer |
2003 |
|||
Brett R. Chapman(6) |
49 |
General Counsel |
2003 |
|||
Carol Hannah(7) |
55 |
President—Distributor Communications and Support |
1984 |
|||
Ken Diekroeger(1) |
41 |
Director |
2002 |
|||
James H. Fordyce(1)(2)(5) |
45 |
Director |
2002 |
|||
Markus Lehmann |
42 |
Director |
2003 |
|||
Charles L. Orr(2) |
61 |
Director |
2002 |
|||
Jesse Rogers(1)(4)(5) |
47 |
Director |
2002 |
|||
Leslie Stanford(4) |
46 |
Director |
2002 |
84
Peter M. Castleman is the Chairman and Managing Partner of Whitney, a position that he has held for more than a decade. Prior to joining Whitney over fifteen years ago, Mr. Castleman was with Morgan Stanley & Co. and prior to that with J.P. Morgan & Co., Inc. Mr. Castleman received his MBA from Harvard Business School and his undergraduate degree from Duke University. Mr. Castleman is currently a director of several private companies. He was previously a director of numerous other companies, including The North Face, Inc., Advance Paradigm, Eon Labs Inc., and Pharmanex, Inc.
Michael O. Johnson is Chief Executive Officer. Mr. Johnson joined Herbalife in April 2003 after 17 years with The Walt Disney Company, where he most recently served as President of Walt Disney International, and also served as President of Asia Pacific for The Walt Disney Company and President of Buena Vista Home Entertainment. Mr. Johnson has also previously served as a publisher of Audio Times magazine, and has directed the regional sales efforts of Warner Amex Satellite Entertainment Company for three of its television channels, including MTV, Nickelodeon and The Movie Channel. Mr. Johnson received his Bachelor of Arts in Political Science from Western State University.
Gregory Probert is Chief Operating Officer of Herbalife. Mr. Probert joined Herbalife in August 2003 after serving as President and CEO of DMX MUSIC for over 2 years. Mr. Probert joined DMX MUSIC after serving as Chief Operating Officer of planetLingo, where he led the team that designed and built the company's first product, an online conversational system for the $20 billion ESL market in Japan. Immediately prior to planetLingo, Mr. Probert spent 12 years with The Walt Disney Company, where he most recently served as Executive Vice President and Chief Operating Officer for the $3.5 billion Buena Vista Home Entertainment worldwide business. Mr. Probert's positions with The Walt Disney Company also included service as Executive Vice President and Managing Director of the International Home Video Division, Senior Vice President and Managing Director of Buena Vista Home Entertainment, Asia Pacific Region, based in Hong Kong, and Chief Financial Officer of Buena Vista International, Disney's theatrical distribution arm, among others. Mr. Probert received his Bachelor of Science from the University of Southern California and his MBA from California State University, Los Angeles.
Henry Burdick is Vice Chairman and in charge of new product development. Mr. Burdick was the co-founder and at various times served as the Chairman, President and CEO of Pharmavite Corporation, the makers of the Nature Made brand of nutritional supplements. In May 1996, following his retirement from Pharmavite, Mr. Burdick invested in a research and development company called Generation Health. He renamed the company Pharmanex, and was Chairman and CEO until it was sold in 1998 to Nu Skin Enterprises, Inc., a NYSE listed company. Mr. Burdick was born in Santiago, Cuba and received a B.A. from California State University, Northridge.
William D. Lowe joined Herbalife in March 1998 and currently is Senior Vice President, Principal Financial and Accounting Officer. In May 2001, Mr. Lowe became the head of Treasury, International Expansion and the Controller's Office. In September 2001, Mr. Lowe's responsibilities were expanded to include financial planning, product pricing and travel and in December 2002 to include taxation. Mr. Lowe started his career with Mobil Corporation and for 23 years held senior management positions responsible for foreign exchange risk management, cash and banking, project financings, mergers and acquisitions, asset sales and employee benefits in New York, Tokyo, London, and Fairfax, Virginia. Mr. Lowe received his Bachelor of Arts in Economics from Georgetown University and his MBA in Finance and International Business from Columbia University.
Matthew Wisk joined Herbalife in July 2003 as Chief Marketing Officer. Mr. Wisk previously served as Vice President of Marketing for Nokia Mobile Phones for North and South America. He also worked for Nokia in Europe, where he headed up marketing for a $7 billion division driving the activities of hundreds of marketing and sales professionals. Prior to Nokia, Mr. Wisk led the marketing for NEC's fax business. Mr. Wisk received his MBA from Michigan State University and began his career with GTE on an executive development program, where his efforts included starting the mobile
85
communications business in Los Angeles, California in the mid 1980s. Mr. Wisk received both his Bachelor of Arts and MBA from Michigan State University.
Brett R. Chapman joined Herbalife in October 2003 as General Counsel. Prior to joining Herbalife, Mr. Chapman spent thirteen years at The Walt Disney Company, most recently as Senior Vice President and Deputy General Counsel, with responsibility for all legal matters relating to Disney's Media Networks Group, including the ABC Television Network, the company's cable properties including The Disney Channel and ESPN, and Disney's radio and internet businesses. Mr. Chapman received his Bachelor of Science and Master of Science in Business Administration from California State University, Northridge and his Juris Doctorate from Southwestern University School of Law.
Carol Hannah joined Herbalife in November 1984 and currently is President—Distributor Communications and Support. Previously she held the position of Co-President, with Brian L. Kane. Ms. Hannah has served in various positions, with primary responsibilities in providing sales and training support for Herbalife's independent distributors. In May 1996, she was promoted to Executive Vice President, Sales Operations and Distributor Services, and in July 2000 to Executive Vice President, Sales, holding that position until September 2002, when she was appointed as Co-President of Herbalife. Prior to joining Herbalife, Ms. Hannah held management positions in the retail apparel and catalog industry.
Ken Diekroeger is a Managing Director of Golden Gate Capital. From 1996 to 2000, Mr. Diekroeger was a managing director and partner with American Industrial Partners. Earlier in his career, Mr. Diekroeger was a consultant at Bain & Company. Mr. Diekroeger received his MBA from Stanford University and his Bachelor of Science in Industrial Engineering from Stanford University. He is currently a director of Stanadyne Automotive Corporation and several private companies.
James H. Fordyce is a partner with Whitney. Prior to joining Whitney, Mr. Fordyce was with Heller Financial and prior to that with Chemical Bank. Mr. Fordyce received his MBA from Fordham University and his undergraduate degree from Lake Forest College. Mr. Fordyce currently is a director of several private companies.
Markus Lehman has been an independent Herbalife distributor for 12 years. A member of the International Chairman's Club, Mr. Lehman is active with distributors of Herbalife's products throughout the world. Mr. Lehman has been active in training other Herbalife Distributors around the world, and has served on various strategy and planning groups for Herbalife. He is involved in various charities including the Herbalife Family Foundation.
Charles L. Orr is an independent director and advisor to companies operating in the e-commerce, financial services and direct selling industries. From 1993 through 2000, Mr. Orr was President and CEO of Shaklee Corporation which included the brand names of Harry and David, Jackson and Perkins and Shaklee. His prior business affiliations include CIGNA, Continental Insurance, Federated Investors, RCA Computer Systems, Southwestern Life and Xerox. Mr. Orr received his MBA from the University of Connecticut and Bachelor of Arts from Wesleyan University. He is an advisor to several private companies, a former director of Provident Mutual Life Insurance Company and currently serves as a board member of the Direct Selling Education Foundation.
Jesse Rogers is a Managing Director of Golden Gate Capital, a San Francisco-based private equity investment firm with approximately $700 million of capital under management. Prior to joining Golden Gate Capital, Mr. Rogers was a partner at Bain & Company for over ten years, where he served as the West Coast head of the consumer products practice and founded Bain & Company's worldwide Private Equity Group. Mr. Rogers received his MBA from Harvard Business School and his Bachelor of Arts from Stanford University. He is currently a director of several private companies and previously served as a director of Beringer Wine Estates and Bain & Company.
Leslie Stanford has been an independent Herbalife distributor for 22 years. A member of the International Chairman's Club, Ms. Stanford is active with distributors of Herbalife's products
86
throughout the world. Ms. Stanford has been active in training other Herbalife distributors around the world, and has served on various strategy and planning groups for Herbalife. She graduated from the University of Alberta, and is involved in various charities including the Herbalife Family Foundation.
Director Compensation
Each independent director receives $25,000 per year for services as a director, plus (1) $5,000 for each Board meeting attended by the director, (2) $2,500 for each committee of the Board on which the director serves, (3) $3,000 per diem for other meetings and (4) reimbursement of all travel and related expenses. Additionally, each independent director was granted options to purchase 50,000 common shares of Holdings at a strike price of $0.44 and options to purchase 50,000 common shares of Holdings at a strike price of $1.76. These options will vest pro rata with 5% vesting on the date of grant and the balance vesting in equal quarterly installments over 19 calendar quarters. In addition the Board granted Henry Burdick options to purchase 300,000 common shares of Holdings at a strike price of $0.44 and options to purchase 300,000 common shares of Holdings at a strike price of $1.76. Both of these grants to Henry Burdick are fully vested.
Directors who are employees of Holdings or any of its affiliates or have been designated as directors by the affiliates of Holdings or its distributors are not independent directors for purposes of director compensation and, in lieu of the compensation described above, receive an annual stipend in the amount of $1,000 for their service as directors.
Executive Compensation
Summary Compensation Table. The following table sets forth the annual and long-term compensation of our Chief Executive Officer and each of the four other most highly compensated
87
executive officers (collectively, the "Named Executive Officers") for the fiscal years ended December 31, 2001, 2002 and 2003.
| |
|
|
|
|
Long-Term Compensation |
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Annual Compensation |
Awards |
|
|
||||||||||||||||||
| Name and Principal Position |
Year |
Salary($) |
Bonus($)(1) |
Other Annual Compensation ($)(2) |
Restricted Stock Award(s)($) |
Securities Underlying Options/ SARs(#) |
LTIP Payouts($) |
All Other Compensation ($)(3) |
|||||||||||||||
| Michael O. Johnson Chief Executive Officer (Joined the Company April 3, 2003) |
2003 | $ | 604,807 | (12) | $ | 1,350,000 | $ | — | $ | — | 5,911,845 | $ | — | $ | 25,790 | (5) | |||||||
Brian L. Kane(4) President, Europe |
2003 2002 2001 |
$ |
712,500 725,384 700,000 |
$ |
425,000 982,500 792,000 |
$ |
— — 60,000 |
$ |
— — — |
1,811,375 — — |
— — — |
$ |
79,091 2,386,977 392,420 |
(6) |
|||||||||
Carol Hannah(4) President Distributor Communications and Support |
2003 2002 2001 |
$ |
712,500 777,885 752,000 |
$ |
425,000 1,054,688 792,000 |
$ |
— — 60,000 |
$ |
— — — |
1,811,375 — — |
— — — |
$ |
35,344 3,435,425 476,305 |
(7) |
|||||||||
Gregory Probert Chief Operating Officer (Joined the Company July 31, 2003) |
2003 |
$ |
207,885 |
(12) |
450,000 |
— |
— |
850,000 |
— |
$ |
6,231 |
||||||||||||
David Kratochvil Chief Logistics Officer |
2003 2002 2001 |
$ |
400,000 425,961 400,000 |
$ |
100,000 125,000 95,385 |
$ |
— — 30,000 |
$ |
— — — |
— 300,000 — |
$ |
— — — |
$ |
31,135 86,428 108,533 |
(9) (10) |
||||||||
John Purdy Senior Vice President Asia/Pacific Rim |
2003 2002 2001 |
$ |
380,000 380,000 350,000 |
$ |
95,000 125,000 74,712 |
$ |
— — 30,000 |
$ |
— — — |
— 300,000 — |
$ |
— — — |
$ |
42,459 178,597 305,032 |
(10) |
||||||||
Robert Levy Senior Vice President The Americas |
2003 2002 2001 |
$ |
380,000 380,000 360,868 |
$ |
95,000 150,000 83,462 |
$ |
— — 30,000 |
$ |
— — — |
— 300,000 — |
$ |
— — — |
$ |
49,766 49,502 37,966 |
(11) |
||||||||
88
89
Option Grants in Last Fiscal Year.
The following table contains information concerning options to purchase common shares of Holdings granted in 2003 to each of the Named Executive Officers. In the judgment of the Board, the per share exercise price of all options described below are higher than the fair market value of Holdings' common shares on the grant date.
| |
Individual Grants |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name |
Number of Securities Underlying Options Granted |
Percent of Total Options Granted to Employees in Fiscal Year |
Exercise Price Per Share($) |
Expiration Date |
Grant Date Present Value ($)(1) |
|||||||
| Michael O. Johnson | 1,182,369 1,182,369 1,182,369 1,182,369 1,182,369 |
7 7 7 7 7 |
% % % % % |
$ |
0.44 1.76 5.28 8.80 12.32 |
4/3/2013 4/3/2013 4/3/2013 4/3/2013 4/3/2013 |
$ |
1,123,251 — — — — |
||||
Brian L. Kane |
1,207,583 603,792 |
7 3 |
% % |
$ |
0.44 1.76 |
3/10/2013 3/10/2013 |
$ |
809,081 |
||||
Carol Hannah |
1,207,583 603,792 |
7 3 |
% % |
$ |
0.44 1.76 |
3/10/2013 3/10/2013 |
$ |
809,081 — |
||||
Gregory Probert |
250,000 150,000 150,000 150,000 150,000 |
1 1 1 1 1 |
% % % % % |
$ |
2.50 3.50 5.50 8.50 11.50 |
7/31/2013 7/31/2013 7/31/2013 7/31/2013 7/31/2013 |
— — — — — |
|||||
David Kratochvil |
— |
— |
— |
— |
— |
|||||||
John Purdy |
— |
— |
— |
— |
— |
|||||||
Robert Levy |
— |
— |
— |
— |
— |
|||||||
90
Aggregated Option Exercises in Last Fiscal Year and Fiscal Year-End Option Values.
The following table sets forth information with respect to: (1) common shares of Holdings acquired upon exercise of stock options and (2) unexercised options to purchase common shares of Holdings granted as of December 31, 2003.
| |
|
|
Securities Underlying Unexercised Options at Fiscal Year-End(#) |
Value of Unexercised In-the-Money Options at Fiscal Year-End ($ in millions)(1) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name |
Shares Acquired on Exercise(#) |
Value Realized($) |
||||||||||||
| Exercisable |
Unexercisable |
Exercisable |
Unexercisable |
|||||||||||
| Michael O. Johnson | — | 5,911,845 | $ | — | $ | 3.5 | ||||||||
| Brian L. Kane | 543,413 | 1,267,962 | $ | 0.9 | $ | 2.2 | ||||||||
| Carol Hannah | 543,413 | 1,267,962 | $ | 0.9 | $ | 2.2 | ||||||||
| Gregory Probert | — | 850,000 | $ | — | $ | — | ||||||||
| David Kratochvil | 75,000 | 225,000 | $ | 0.1 | $ | 0.3 | ||||||||
| John Purdy | 75,000 | 225,000 | $ | 0.1 | $ | 0.3 | ||||||||
| Robert Levy | 75,000 | 225,000 | $ | 0.1 | $ | 0.3 | ||||||||
Description of Benefit Plans
WH Holdings (Cayman Islands) Ltd. Stock Incentive Plan. Holdings has established a stock incentive plan that provides for the grant of options to purchase common shares of Holdings or stock appreciation rights to employees or consultants of Herbalife. The purpose of the plan is to promote the long-term financial interest and growth of the Company by attracting and retaining employees and consultants who can make a substantial contribution to the success of the Company, to motivate and to align interests with those of the equity holders.
Each stock option agreement and SAR award agreement will specify the date when all or any installment of an award is to become exercisable but, generally, no award may be exercisable after the expiration of 10 years from the date it was granted. Upon termination of employment for any reason other than "cause," the unvested awards would continue to be exercisable for a period of time, following which the award will terminate.
Upon termination of employment of a senior executive, Holdings may repurchase the shares if such termination: (i) was due to resignation or for cause (A) before the seventh anniversary of the date of option grant, at an amount equal to the lesser of: (a) the fair market value at the time of such repurchase and (b) the exercise price, and (B) after the seventh anniversary of the date of option grant, at an amount equal to the fair market value at the time of such repurchase; or (ii) was involuntary without cause or because of death, retirement or disability at an amount equal to the greater of: (a) the fair market value at the time of such repurchase and (b) the exercise price.
Upon termination of employment of an employee other than a senior executive, Holdings will have the right to repurchase the shares acquired upon the exercise of options within a specified period at a price equal to either (a) the fair market value of the shares at the time of repurchase or (b) the exercise price, however, the right to repurchase at the exercise price lapses at the rate of 20% for each full grant year.
WH Holdings (Cayman Islands) Ltd. Independent Directors Stock Option Plan. Holdings has established an independent directors stock option plan that provides for the grant of options to purchase common shares of Holdings to independent directors of Holdings. Directors who are employees of Holdings or any of its affiliates or have been designated as directors by the affiliates of Holdings or its distributors are not independent directors for purposes of director compensation.
91
The purpose of the plan is to promote the long-term financial interest and growth of the Company by attracting and retaining independent directors who can make a substantial contribution to the success of the Company, to motivate and to align interests with those of the equity holders. The option plan is administered by the compensation committee. One million shares have been reserved for grant under this plan.
Approximately 15.5% of the share capital at the time of the Merger or 18.7 million shares of Holdings are available for grant under the two plans. As of December 31, 2003, the Company had granted approximately 17.7 million stock options to eligible employees equal to 17.4% of the share capital of Holdings, of which 0.8 million were under the Independent Directors Plan.
Upon termination of an independent director, Holdings and the institutional shareholders have the right to repurchase the shares if such termination (i) was voluntary, due to resignation or for cause at an amount equal to the lesser of: (a) the fair market value at the time of such termination; or (b) the exercise price; (ii) was involuntary without cause or because of death, retirement or disability at an amount equal to the greater of: (a) the fair market value at the time of such termination; or (b) the exercise price.
Deferred Compensation Plans. We maintain three deferred compensation plans for select groups of management or highly compensated employees: (1) the Herbalife Management Deferred Compensation Plan, effective January 1, 1996 (the "Management Plan"), which is applicable to directors and vice presidents; (2) the Herbalife Senior Executive Compensation Plan, effective January 1, 1996 (the "Senior Executive Plan"), which is applicable to eligible employees at the rank of Senior Vice President and higher and (3) the Supplemental Senior Executive Deferred Compensation Plan (the "Supplemental Plan") effective July 30, 2002. The Management Plan and the Senior Executive Plan are referred to as the "Deferred Compensation Plans." The Deferred Compensation Plans were amended and restated effective January 1, 2001.
The Deferred Compensation Plans are unfunded and benefits are paid from our general assets, except that we have contributed amounts to a "rabbi trust" whose assets will be used to pay benefits if we remain solvent, but can be reached by our creditors if we become insolvent. The Deferred Compensation Plans allow eligible employees, who are selected by the administrative committee that manages and administers the plans (the "Deferred compensation committee"), to elect annually to defer up to 50% of their annual base salary and up to 100% of their annual bonus for each calendar year (the "Annual Deferral Amount"). We make matching contributions on behalf of each participant in the Senior Executive Plan ("Matching Contributions").
Effective January 1, 2002, the Senior Executive Plan was amended to provide that the amount of the Matching Contributions is to be determined by us in our discretion. For 2002 the Matching Contribution was equal to an amount of up to 7.5% of a participant's annual base salary. Effective January 1, 2003, the Matching Contribution has been reduced to 3% and remains 3% for 2004.
Each participant in a Deferred Compensation Plan may determine how his or her Annual Deferral Amount and Matching Contributions, if any, will be deemed to be invested by choosing among several investment funds or indices designated by the Deferred compensation committee. The Deferred Compensation Plans, however, do not require us to actually acquire or hold any investment fund or other assets to fund the Deferred Compensation Plans. The entire interest of each participant in a Deferred Compensation Plan is always fully vested and non-forfeitable.
In connection with a participant's election to defer an Annual Deferral Amount, the participant may also elect to receive a short-term payout, equal to the Annual Deferral Amount and the Matching Contributions, if any, attributable thereto plus earnings, and shall be payable two or more years from the first day of the year in which the Annual Deferral Amount is actually deferred. As of January 2004, the Deferred Compensation Plans were amended to allow for deferral of the short-term payout date if the deferral is made within the time period specified therein. Subject to the short-term payout provision and specified exceptions for unforeseeable financial emergencies, a participant may not
92
withdraw, without incurring a ten percent (10%) withdrawal penalty, all or any portion of his or her account under the Deferred Compensation Plans prior to the date that such participant either (1) is determined by the Deferred compensation committee to have incurred permanent and total disability or (2) dies or otherwise terminates employment.
The Supplemental Plan is unfunded and all benefits thereunder are paid from our general assets, except that we have contributed amounts to a "rabbi trust" whose assets will be used to pay benefits if we remain solvent, but can be reached by our creditors if we become insolvent. The Supplemental Plan allows employees to participate who are highly compensated and who are eligible to participate in the Herbalife International, Inc. Senior Executive Change in Control Plan (the "Change in Control Plan"). The Deferred compensation committee allows eligible employees to defer up to 100% of their Change in Control Payment. A "Change in Control Payment" is an amount equal to three times an eligible employee's compensation.
Each participant in the Supplemental Plan will be deemed to have invested in funds that provide a return equal to the short-term applicable federal rate, within the meaning of the Internal Revenue Code of 1986, as amended (the "Code"). The Supplemental Plan, however, does not require us to actually acquire or hold any investment fund or other assets to fund the Supplemental Plan. The entire interest of each participant in a Supplemental Plan is always fully vested and non-forfeitable. In connection with a participant's election to defer the Change in Control Payment, the participant may also elect to receive a short-term payout, equal to the deferral amount plus earnings and payable two or more years from the first day of the year in which the deferral amount is actually deferred. Subject to the short-term payout provision and specified exceptions for unforeseeable financial emergencies, a participant may not withdraw, without incurring a ten percent (10%) withdrawal penalty, all or any portion of his or her account under the Supplemental Plan prior to the date that such participant either (1) is determined by the Deferred compensation committee to have incurred permanent and total disability or (2) dies or otherwise terminates employment.
Executive Retention Plan. We have an Executive Retention Plan. The purpose of the Executive Retention Plan is to provide financial incentives for a select group of management and highly compensated employees of the Company to continue to provide services to the Company during the period immediately before and immediately after change in control, as defined.
As a result of certain actions by Herbalife's Board, the Acquisition was not deemed to be a Change in Control under the Executive Retention Plan. Thus, the consummation of the Acquisition did not result in the payment of any benefit pursuant to the Executive Retention Plan.
We also established an Executive Retention Trust to provide benefits under the Executive Retention Plan. The Executive Retention Trust is an irrevocable trust established with an institutional trustee. This irrevocable trust's assets will be used to pay the benefits of the Executive Retention Plan and are not intended to be reachable by our creditors. The value of the assets in the irrevocable trust was $2.8 million as of March 31, 2004. The Administrative Committee of the Executive Retention Plan will establish an individual account in the Executive Retention Trust for each participant in the Executive Retention Plan. Until the occurrence of a change in control, the Administrative Committee will control the investment of the assets in the Executive Retention Trust, and will determine the allocation of the assets of the Executive Retention Trust to the individual accounts of participants. Each participant who qualifies for a benefit under the Executive Retention Plan will receive a lump sum benefit equal to the dollar amount in his or her individual account in the Executive Retention Trust. The benefit shall be paid within 90 days after the participant qualifies for the benefit. If a participant's employment with the Company terminates before the participant qualifies for a benefit under the Executive Retention Plan, the participant's account in the Executive Retention Trust will revert to the Company. A participant's benefit under the Executive Retention Plan will be reduced if the amount would cause payment of federal excise tax.
93
401(k) profit sharing plan. We maintain a tax-qualified profit sharing plan pursuant to Sections 401(a) and 401(k) of the Code (the "401(k) Plan"). The 401(k) Plan allows any eligible employee, including specified common-law employees, to contribute each pay period from 2% to 17% of the employee's earnings (but not in excess of $13,000 per year, as adjusted after 2003) or $16,000 in the case of those participants over 50 years of age for investment in mutual funds held by the 401(k) Plan's trust. The Company makes contributions to the 401(k) Plan in an amount equal to 3% of the earnings of each employee who elects to defer 2% or more of his or her earnings and beginning on January 1, 2003 a matching contribution equal to one dollar for each dollar of deferred earnings not to exceed 3% of the participant's earnings. The 401(k) Plan also imposes restrictions on the aggregate amount that may be contributed by higher-paid employees in relation to the amount contributed by the remaining employees. A participating employee is fully vested at all times in his or her contributions and in the trust fund's earnings attributable to his or her contributions. An employee becomes fully vested in the Company's contributions and earnings of the trust fund attributable to the Company's contributions (1) upon the employee's death, (2) upon the employee's disability, or (3) upon the employee reaching the 401(k) Plan's normal retirement age, which is the latter of age 65 and the completion of five years of service with the Company. An employee may not withdraw all or any portion of his or her account prior to the date that the employee either (1) incurs a hardship or (2) terminates employment with the Company. Effective January 1, 2003, the 401(k) Plan was amended to provide that an employee vests in 20% increments annually until fully vested upon the fifth anniversary of his participation in the 401(k) Plan.
Employment Contracts
On April 3, 2003, we announced the appointment of Mr. Michael O. Johnson as Chief Executive Officer and director. Our subsidiaries, Herbalife and Herbalife International of America, Inc. ("Herbalife America") entered into an executive employment agreement (the "Johnson Employment Agreement") with Mr. Johnson effective as of April 3, 2003. For his services, Mr. Johnson is entitled to receive an annual salary of $850,000. Under the terms of the Johnson Employment Agreement, in addition to his salary, Mr. Johnson shall be entitled to participate in or receive benefits under each benefit plan or arrangement made available by the us to our senior executives on terms no less favorable than those generally applicable to senior executives of Herbalife and Herbalife America.
Mr. Johnson is also eligible to receive an annual cash bonus in such amounts, and based on such targets, established annually by the Board of Directors in accordance with the Johnson Employment Agreement. Mr. Johnson's annual bonus for the fiscal year ending December 31, 2003 was $1,350,000 and was dependent, in part, on our operating subsidiaries' 2003 EBITDA performance.
In addition, Mr. Johnson has been granted stock options under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan to purchase an aggregate of 5,911,845 common shares of Holdings at exercise prices as follows: 1,182,369 shares at $0.44 per share, 1,182,369 shares at $1.76 per share, 1,182,369 shares at $5.28 per share, 1,182,369 shares at $8.80 per share, and 1,182,369 shares at $12.32 per share. The options vest under a schedule over time through June 30, 2008. The options expire 10 years after the date of grant.
In the event of any Change of Control (as defined in the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan), 50% of the shares granted pursuant to the options (pro rata according to the number of shares exercisable at the relevant exercise prices specified above for each of the individual tranches) will become immediately vested and exercisable. If, following any Change of Control, all or any portion of the options remain outstanding and Mr. Johnson's employment is terminated (other than by reason of Mr. Johnson's resignation without Good Reason or termination by us for Cause (each as defined in the Johnson Employment Agreement)) at any time following such Change of Control, 100% of the shares granted pursuant to the options will immediately vest and become exercisable. In the event Mr. Johnson's employment is terminated by reason of Mr. Johnson's
94
death or disability or during the 90 day period before any Change of Control, 100% of the shares granted pursuant to the options will immediately vest and become exercisable.
We have also entered into an executive employment agreement (the "Probert Employment Agreement") effective July 31, 2003 with Mr. Gregory Probert through our subsidiary Herbalife America. Pursuant to the Probert Employment Agreement, Mr. Probert served as Executive Vice President until December 31, 2003 and as Chief Operating Officer thereafter. The term of the Probert Employment Agreement expires on August 11, 2006. For his services as Executive Vice President, Mr. Probert was compensated at a pro-rated salary of $525,000 per annum. Starting on January 1, 2004, for his services as Herbalife America's Chief Operating Officer, Mr. Probert is entitled to receive an annual salary of $680,000. Under the terms of the Probert Employment Agreement, in addition to his salary, Mr. Probert is entitled to participate in or receive benefits under each benefit plan or arrangement made available by us to our senior executives on terms no less favorable than those generally applicable to senior executives of Herbalife America.
In addition, Mr. Probert received an annual cash bonus of $450,000 for the fiscal year ending December 31, 2003 and is eligible to receive an annual cash bonus equal to 100% of the applicable annual bonus thereafter, calculated in accordance with the then-current bonus formula approved by us for our most senior officers.
Mr. Probert has also been granted stock options under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan to purchase an aggregate of 850,000 common shares of Holdings at exercise prices as follows: 250,000 shares at $2.50 per share, 150,000 shares at $3.50 per share, 150,000 shares at $5.50 per share, 150,000 shares at $8.50 per share, and 150,000 shares at $11.50 per share. The options vest under a schedule over time through July 31, 2008. The options expire 10 years after the date of grant.
In the event of any Change of Control (as defined in the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan), 50% of the shares granted pursuant to the options (pro rata according to the number of shares exercisable at the relevant exercise prices specified above for each of the individual tranches) will become immediately vested and exercisable. If, following any Change of Control, all or any portion of the options remain outstanding and Mr. Probert's employment is terminated (other than by reason of Mr. Probert's resignation without Good Reason or termination by us for Cause at any time following such Change of Control, 100% of the shares granted pursuant to the options will immediately vest and become exercisable. In the event Mr. Probert's employment is terminated by reason of Mr. Probert's death or disability or during the 90 day period before any Change of Control, 100% of the shares granted pursuant to the options will immediately vest and become exercisable.
We have also entered into an executive employment agreement (the "Goudis Employment Agreement") effective June 1, 2004 with Mr. Richard Goudis through our subsidiary Herbalife America. Pursuant to the Goudis Employment Agreement, Mr. Goudis will serve as Chief Financial Officer beginning June 14, 2004. For his services as Chief Financial Officer, Mr. Goudis will be entitled to a salary of $430,000 for his first full calendar year of employment, $475,000 for his second year, and $500,000 for his third year. Under the terms of the Goudis Employment Agreement, in addition to his salary, Mr. Goudis is entitled to participate in or receive benefits under each benefit plan or arrangement made available by us to our senior executives on terms no less favorable than those generally applicable to senior executives of Herbalife America.
In addition, Mr. Goudis will be eligible to receive an annual cash bonus equal to 50% of his then-current base salary, calculated in accordance with the then-current bonus formula approved by us for our most senior officers.
We also agreed to grant to Mr. Goudis options under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan to purchase an aggregate of 400,000 common shares of Holdings at exercise prices as follows: 80,000 shares at $4.01 per share; 80,000 shares at $6.00 per share; 80,000
95
shares at $8.00 per share; 80,000 shares at $10.00 per share; and 80,000 shares at $12.00 per share. The options vest at the rate of 5% per calendar quarter. The options expire 10 years after the date of grant.
Mr. Burdick is an at-will employee and for his services as Vice Chairman, Mr. Burdick is entitled to receive an annual salary of $500,000. In addition, Mr. Burdick is eligible to receive a discretionary bonus. For 2003 the bonus was zero.
Mr. Burdick was granted 50,000 options to purchase Holdings common shares at an exercise price of $0.44 per share and 50,000 options to purchase Holdings common shares at an exercise price of $1.76 per share under the WH Holdings (Cayman Islands) Ltd. Independent Directors Stock Option Plan, of which 30,000 were exercisable within 60 days of December 31, 2003. In addition the Board granted Mr. Burdick options to purchase 300,000 common shares of Holdings at a strike price of $0.44 and options to purchase 300,000 common shares of Holdings stock at a strike price of $1.76 under the WH Holdings (Cayman Islands) Ltd. Independent Directors Stock Option Plan. These 600,000 options have vested. In 2003, Mr. Burdick accepted an executive management position with us and now serves as our Vice Chairman. As a result, Mr. Burdick may no longer be considered an independent director. Under the WH Holdings (Cayman Islands) Ltd. Independent Directors Stock Option Plan, the termination of Mr. Burdick as an independent director results in the unexercisable portion of the options granted pursuant to the plan terminating on the date of such termination and the remaining exercisable portion of the options granted pursuant to the plan becoming exercisable for thirty days following termination as an independent director. In light of the fact that the termination of Mr. Burdick's status as an independent director occurred at the request of the Board, in 2003, the Compensation Committee of the Board took action to waive those provisions that would have resulted in the termination of the unexercisable portion of Mr. Burdick's options granted under the plan and that would have caused the remaining exercisable portion of those options to become exercisable for only thirty days following the termination of his status as an independent director.
In connection with the engagement of Mr. Burdick as Vice Chairman, Mr. Burdick was granted an aggregate of 400,000 options to purchase common shares of Holdings under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan at exercise prices as follows: 80,000 shares at $0.44 per share, 80,000 shares at $1.76 per share, 80,000 shares at $5.28 per share, 80,000 shares at $8.80 per share, and 80,000 shares at $12.32 per share. The options vest under a schedule over time through June 30, 2008. The options expire 10 years after the date of grant.
In the event of any Change of Control (as defined in the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan), 50% of the shares granted pursuant to the options (pro rata according to the number of shares exercisable at the relevant exercise prices specified above for each of the individual tranches) issued to Mr. Burdick under that plan will become immediately vested and exercisable. If, following any Change of Control, all or any portion of the options issued to Mr. Burdick under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan remain outstanding and Mr. Burdick's employment is terminated (other than by reason of Mr. Burdick's resignation without Good Reason or termination by us for Cause) at any time following such Change of Control, 100% of the shares granted pursuant to the options issued to Mr. Burdick under that plan will immediately vest and become exercisable. In the event Mr. Burdick's employment is terminated by reason of Mr. Burdick's death or disability or during the 90 day period before any Change of Control, 100% of the shares granted pursuant to the options issued to Mr. Burdick under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan will immediately vest and become exercisable.
Mr. Lowe is an at-will employee and for his services as Senior Vice President, Principal Financial and Accounting Officer, Mr. Lowe is entitled to receive an annual salary of $350,000. In addition, Mr. Lowe is eligible to receive a target bonus in the amount of 35% of Mr. Lowe's annual salary.
Mr. Lowe was granted an aggregate of 300,000 options to purchase common shares of Holdings under the WH Holdings (Cayman Islands) Ltd. Stock Incentive Plan at exercise prices as follows:
96
150,000 shares at $0.44 per share and 150,000 shares at $1.76 per share. The options vest under a schedule over time through October 22, 2007. The options expire ten (10) years after the date of grant.
We have also entered into an executive employment agreement (the "Wisk Employment Agreement") with Mr. Matt Wisk through our subsidiary Herbalife America, pursuant to which Mr. Wisk will serve as Chief Marketing Officer, effective as of July 21, 2003. The term of the Wisk Employment Agreement expires on July 21, 2005, at which time Mr. Wisk shall be employed on an at-will basis. For his services, Mr. Wisk is entitled to receive an annual salary of $388,000. Under the terms of the Wisk Employment Agreement, in addition to his salary, Mr. Wisk was entitled to reimbursement for relocation expenses, in accordance with our past practice of reimbursing executives for such reasonable and customary expenses. Additionally, Mr. Wisk shall be entitled to participate in or receive benefits under each benefit plan or arrangement made available by us to our senior executives on terms no less favorable than those generally applicable to senior executives of Herbalife America.
Mr. Wisk received an annual cash bonus of $150,000 for the fiscal year ending December 31, 2003, and is eligible to receive an annual cash bonus equal to 50% of Mr. Wisk's annual salary thereafter. He has also been granted a non-qualified stock option grant under the WH Holdings (Cayman Islands) Ltd. Stock Option Plan to purchase an aggregate of 300,000 common shares of Holdings at exercise prices as follows: the first 60,000 shares at $2.50 per share, the second 60,000 shares at $3.50 per share, the third 60,000 shares at $5.50 per share, the fourth 60,000 shares at $8.50 per share, and the fifth 60,000 shares at $11.50 per share. The options vest under a schedule over time through July 14, 2008. The options expire 10 years after the date of grant.
Unless otherwise approved by a written resolution of the Committee (as defined in the WH Holdings (Cayman Islands) Ltd. Stock Option Plan) prior to or contemporaneously with the closing of any such transaction, any portion of the options granted to Mr. Wisk under that plan (whether vested or unvested and whether or not then exercisable) which has not been exercised prior to or in connection with any merger or consolidation of Holdings into another corporation, the exchange of all or substantially all of the assets of Holdings for the securities of another corporation, a Change of Control or the recapitalization, reclassification, liquidation or dissolution of Holdings or any other fundamental corporate transaction involving Holdings or any of its subsidiaries with the same or a similar purpose or effect shall expire and be cancelled and of no further force and effect effective upon the closing of any such transaction.
Before July 21, 2005, if Mr. Wisk terminates his employment by reason of Mr. Wisk's death, disability or for "good reason" as defined in the Wisk Employment Agreement, or he is terminated by us with or without cause, Mr. Wisk will receive $680,000 less the amount of salary already received. If such termination occurs prior to July 21, 2004, Mr. Wisk will receive an additional payment of $85,000. During the at-will employment period after July 21, 2005, if Mr. Wisk terminates his employment by reason of Mr. Wisk's death, disability or for "good reason" or he is terminated by us, Mr. Wisk will receive a minimum six-month severance payment calculated at Mr. Wisk's then-current salary, in an amount not less than $170,000.
On October 6, 2003, we appointed Mr. Brett R. Chapman as General Counsel. We have entered into an executive employment agreement (the "Chapman Employment Agreement") with Mr. Chapman effective as of October 6, 2003 through our subsidiary, Herbalife America. For his services, Mr. Chapman is entitled to receive an annual salary of $435,000. Under the terms of the Chapman Employment Agreement, in addition to his salary, Mr. Chapman shall be entitled to participate in or receive benefits under each benefit plan or arrangement made available by us to our senior executives on terms no less favorable than those generally applicable to senior executives of Herbalife America.
In addition, Mr. Chapman received an annual cash bonus of $140,000 for the fiscal year ending December 31, 2003 and is eligible to receive an annual cash bonus equal to 100% of the applicable annual bonus thereafter, calculated in accordance with the then-current bonus formula approved by us
97
for our most senior officers. Mr. Chapman's target bonus is set in the Chapman Employment Agreement at an amount equal to 50% of Mr. Chapman's annual salary for the year with respect to which the bonus is to be paid.
Mr. Chapman has also been granted stock options under the WH Holdings (Cayman Islands) Ltd. Option Plan to purchase an aggregate of 325,000 common shares of Holdings at exercise prices as follows: 150,000 shares at $2.50 per share, 43,750 shares at $3.50 per share, 43,750 shares at $5.50 per share, 43,750 shares at $8.50 per share, and 43,750 shares at $11.50 per share. The options vest under a schedule over time through October 6, 2008. The options expire 10 years after the date of grant.
In the event of any Change of Control (as defined in the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan), 50% of the shares granted pursuant to the options (pro rata according to the number of shares exercisable at the relevant exercise prices specified above for each of the individual tranches) issued to Mr. Chapman under that plan will become immediately vested and exercisable. If, following any Change of Control, all or any portion of the options issued to Mr. Chapman under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan remain outstanding and Mr. Chapman's employment is terminated (other than by reason of Mr. Chapman's resignation without Good Reason or termination by us for Cause (as defined in the Chapman Employment Agreement)) at any time following such Change of Control, 100% of the shares granted pursuant to the options issued to Mr. Chapman under that plan will immediately vest and become exercisable. In the event Mr. Chapman's employment is terminated by reason of Mr. Chapman's death or disability or during the 90 day period before any Change of Control, 100% of the shares granted pursuant to the options issued to Mr. Chapman under the WH Holdings (Cayman Islands) Ltd. Executive Officer Stock Option Plan will immediately vest and become exercisable.
We have also entered into an executive employment agreement (the "Hannah Employment Agreement") with Carol Hannah through our subsidiaries Herbalife and Herbalife America. The Hannah Employment Agreement became effective as of March 10, 2003. The Hannah Employment Agreement is for a three year term. Ms. Hannah is engaged as President of Distributor Communications and Support. For her services, Ms. Hannah is entitled to receive an annual salary of $712,500. Under the terms of the Hannah Employment Agreement, in addition to her salary, Ms. Hannah shall be entitled to participate in or receive benefits under each benefit plan or arrangement made available by us to our senior executives on terms no less favorable than those generally applicable to senior executives of Herbalife and Herbalife America.
Under the terms of the Hannah Employment Agreement, if, at any time during the term of the Hannah Employment Agreement, (1) Herbalife terminates Ms. Hannah's employment without Cause (as defined in the Hannah Employment Agreement) Herbalife must pay Ms. Hannah (in addition to all accrued base salary, bonus for the year preceding the year of termination, benefits and other amounts Ms. Hannah is entitled to) an amount equal to one year's salary and bonus (the bonus for the year of termination shall be equal to one year's base salary). In addition, Herbalife shall continue to afford to Ms. Hannah medical, dental, vision, long-term disability and life insurance benefits for one year. If Ms. Hannah (1) dies or (2) becomes disabled at any time during the term of the Hannah Employment Agreement, upon the Death or Disability of Ms. Hannah (as defined in the Hannah Employment Agreement), Herbalife must pay Ms. Hannah or her beneficiaries or estate (in addition to all accrued base salary, bonus for the year preceding the year of termination, benefits and other amounts Ms. Hannah is entitled to) Ms. Hannah's base salary and bonus for one year (the bonus for the year of termination shall be equal to one year's base salary). In the event Ms. Hannah terminates her employment or Herbalife terminates Ms. Hannah's employment for Cause, Herbalife must pay Ms. Hannah all accrued base salary, bonus for the year preceding the year of termination, benefits and other amounts Ms. Hannah is entitled to.
Ms. Hannah has been granted stock options as of March 10, 2003 under the WH Holdings (Cayman Islands) Ltd. Option Plan to purchase 1,207,583 common shares of Holdings at an exercise
98
price of $0.44 per share and 603,792 common shares of Holdings at an exercise price of $1.76 per share. The options granted to Ms. Hannah are subject to a vesting schedule whereby 15% of the options vest immediately and thereafter, vest at a rate of 5% each quarter until all of the options become fully vested and exercisable as of June 30, 2007. The options expire 10 years after the date of grant.
Under the terms of the stock option grants, in the event Ms. Hannah's employment with Herbalife is terminated for whatever reason, the unexercisable portion of Ms. Hannah's stock options will terminate on the date of such termination and the exercisable portion of Ms. Hannah's stock options will be treated as follows. Subject to Herbalife's right to repurchase the shares and subject to the shareholders' agreement, if Ms. Hannah's employment is terminated for Cause, the exercisable portion of Ms. Hannah's stock options will terminate on the date of such termination. If Ms. Hannah's employment is terminated for any reason except for Cause, the exercisable portion of Ms. Hannah's stock options will be exercisable for 30 days following the termination. If Ms. Hannah's employment is terminated on account of a "disability" as defined in Section 22(e) of the Code or death, Ms. Hannah or Ms. Hannah's personal representative may exercise the exercisable portion of Ms. Hannah's stock options for 90 days following the termination of employment on account of such disability or Ms. Hannah's death. In addition, in connection with certain transaction involving a change in control (as defined in the stock option agreement) or the initial public offering of Herbalife's common shares, the previously unexercisable portion of Ms. Hannah's stock options will immediately become 100% vested and exercisable immediately prior to the closing of any such transaction.
Ms. Hannah recently notified us of her intention to retire from the Company. As a result, we entered into an amicable separation agreement and general release with her (the "Separation Agreement"), pursuant to which we agreed to terminate the Hannah Employment Agreement and to provide for certain mutual releases of claims, effective as of June 30, 2004. In addition, the Separation Agreement provides that while Ms. Hannah shall remain employed pursuant to the Hannah Employment Agreement through June 30, 2004, from and after May 1, 2004, she will no longer be required to render her normal services for the Company except that she must be available at our reasonable request to provide advice and counsel not to exceed twenty hours per month. In addition, we entered into a consulting agreement with Ms. Hannah to engage her as an independent contractor to consult on all aspects of the Company's business through April 30, 2006. For her services, Ms. Hannah will receive a consulting fee of $59,375 per month during the term of the consultancy.
Compensation Committee Interlocks and Insider Participation
From January 1 through December 31, 2003, the Compensation Committee consisted of Messrs. Jesse Rogers, James Fordyce, Steven Rodgers, and Ken Diekroeger. Steven Rodgers was an officer of Holdings from April 2002 through the end of the last fiscal year.
Compensation Committee Report
Herbalife's executive compensation programs are administered by the Compensation Committee and, to the extent summarized below, our Chief Executive Officer or the person performing similar functions.
The compensation policy is designed to motivate the overall success of the Company by:
99
Base Salary and Annual Incentive Compensation
Mr. Johnson's base salary beginning April 3, 2003 was established through negotiations between him and the Compensation Committee. The Compensation Committee believes Mr. Johnson's compensation should be heavily influenced by the Company's performance. In evaluating Mr. Johnson's compensation, the Compensation Committee considered the financial results of the Company, the compensation (including base salary, annual and long-term incentives) paid to Mr. Johnson by his previous employer, the compensation (including base salary, annual and long-term incentives) paid to executives in similar positions in similar industries, Mr. Johnson's reputation, leadership experience and leadership skills, and the compensation paid to other senior executives of the Company. As discussed above with respect to the payment of performance bonuses, Mr. Johnson received a bonus for fiscal 2003 in an amount equal to that required under the formula specified in the Johnson Employment Agreement plus an additional discretionary amount which, in the Compensation Committee's opinion, reflected the progress the Company made during fiscal 2003.
Base salaries for each of the other Named Executive Officers were established through negotiations between Mr. Johnson or his predecessor and each such Named Executive Officer. See the Summary Compensation Table under "—Executive Compensation."
Long-Term Incentive Plans
Our executives are encouraged to own common shares of Holdings, issuable upon exercise of stock options, thereby aligning the interests of management with those of shareholders and tying a significant portion of executive compensation to long-term performance. The vesting schedules for stock options are set by the Compensation Committee. All options granted to executive officers have had an exercise price of more than or equal to 100% of fair market value on the date of grant.
Tax Issues
The Compensation Committee intends to seek to structure executive compensation arrangements to maximize the deductibility of Named Executive Officer compensation under applicable federal and state tax laws, including the Omnibus Budget Reconciliation Act of 1993, while also taking into account the need to provide appropriate incentives to our key executives. However, no assurance can be given that we will preserve, or will seek to preserve, the deductibility of all executive compensation.
COMPENSATION COMMITTEE
Jesse
Rogers
James Fordyce
Ken Diekroeger
June 9, 2004
100
Whitney V, L.P. and Whitney Strategic Partners V, L.P. (together with certain affiliated investment funds) and CCG Investments (BVI), L.P. (together with certain of its co-investment funds), as well as selected members of our distributor organization and our management are the owners of all of the outstanding capital stock of Holdings. The address for Whitney V, L.P. and Whitney Strategic Partners V, L.P. is c/o Whitney & Co., LLC, 177 Broad Street, Stamford, Connecticut 06901. The address for CCG Investments (BVI), L.P. is c/o Golden Gate Private Equity, Inc., One Embarcadero Center, 33rd Floor, San Francisco, California 94111.
Prior to the consummation of the Transactions, Holdings' outstanding securities consisted of 102 million shares of its 12% Series A Cumulative Convertible Preferred Shares, par value $0.001 per share. After the consummation of the Transactions, Holdings' outstanding securities consists of 104.1 million Common Shares, par value $0.001 per share, each share being entitled to one vote on matters submitted to shareholders' vote.
Management participates in our equity through option grants by Holdings under a stock incentive plan. See "Certain Relationships and Related Transactions—Certain Transactions Relating to Holdings—WH Holdings (Cayman Islands) Ltd. Stock Incentive Plan."
The following table shows the beneficial ownership of common shares of Holdings as of March 31, 2004, and thus the indirect beneficial ownership of the equity interest of Herbalife as of that date, held by (i) each of Holdings' and Herbalife's directors, (ii) each of the five mostly highly compensated executive officers of Herbalife, (iii) all directors and executive officers as a group and (iv) each person or entity known to Herbalife to beneficially own more than five percent (5%) of the outstanding common shares of Holdings.
101
| Name and address of beneficial owner |
Number of shares |
Percentage ownership on a fully diluted basis(1) |
|||
|---|---|---|---|---|---|
| Whitney V, L.P.** | 52,032,570 | 50.0 | % | ||
| Whitney Strategic Partners V, L.P.** | 456,460 | * | |||
| Whitney Private Debt Fund, L.P.** | 805,585 | * | |||
| Green River Offshore Fund** | 85,929 | * | |||
| Total | 53,380,544 | 51.3 | % | ||
CCG Investments (BVI), L.P.*** |
26,454,793 |
25.4 |
% |
||
| CCG Associates—QP, LLC*** | 1,329,857 | 1.3 | % | ||
| CCG Associates—AI, LLC*** | 123,654 | * | |||
| CCG Investment Fund—AI, LP*** | 354,406 | * | |||
| CCG AV, LLC—Series C*** | 872,712 | * | |||
| CCG AV, LLC—Series E*** | 708,836 | * | |||
| CCG CI*** | 452,484 | * | |||
| Total | 30,296,742 | 29.1 | % | ||
Peter M. Castleman(2)** |
53,380,544 |
51.3 |
% |
||
| James H. Fordyce** | 0 | * | |||
| John C. Hockin(2)(14)** | 52,489,030 | 50.4 | % | ||
| Steven E. Rodgers(2)(14)** | 52,489,030 | 50.4 | % | ||
| Jesse Rogers(3)*** | 30,296,742 | 29.1 | % | ||
| Prescott Ashe(3)(14)*** | 30,296,742 | 29.1 | % | ||
| Ken Diekroeger(3)*** | 30,296,742 | 29.1 | % | ||
| Stefan L. Kaluzny(14)*** | 0 | * | |||
| Leslie Stanford(4)**** | 2,582,955 | 2.5 | % | ||
| Markus Lehman**** | 1,105,682 | 1.1 | % | ||
| Charles L. Orr(5)**** | 49,205 | * | |||
| Henry Burdick(6)**** | 1,307,181 | 1.3 | % | ||
| Michael O. Johnson(7)**** | 1,740,701 | 1.7 | % | ||
| Carol Hannah(8)**** | 1,202,163 | 1.2 | % | ||
| Brian L. Kane(9)**** | 918,072 | * | |||
| Gregory Probert(10)**** | 0 | * | |||
| David Kratochvil(11)**** | 118,409 | * | |||
| John B. Purdy(12)**** | 140,000 | * | |||
| Robert Levy(13)**** | 118,409 | * | |||
| All Directors and Executive Officers as a Group (19 persons) | |||||
| Total | 92,960,063 | 86.1 | % |
102
the knowledge of Herbalife, all persons listed below have sole voting and investment power with respect to their common shares, except to the extent authority is shares by spouses under applicable law and to the extent provided in the shareholders' agreement. See "Certain Relationships and Related Transactions—Certain Transactions Relating to Holdings—Shareholders' Agreement." Pursuant to the rules of the Securities and Exchange Commission, the number of shares of Common Stock deemed outstanding includes shares issuable pursuant to options or warrants held by the respective person or group which may be exercised within 60 days of March 31, 2004.
103
exercise price of $12.32 per share of which 1,537,081 are exercisable within 60 days of March 31, 2004.
104
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Redemption of Preferred Shares
A portion of the proceeds from the offering of the Outstanding Notes was applied to pay the cash redemption price for all of Holdings' outstanding Preferred Shares. To permit Holdings to convert the Preferred Shares, we amended Holdings' charter documents to permit its Board of Directors to elect to convert all of the outstanding Preferred Shares into the right to receive a cash payment, for each Preferred Share converted, equal to the original issue price for the Preferred Shares ($1.76 per share), and all accrued and unpaid dividends, plus one common share of Holdings. In connection with the consummation of the Transactions described herein, all of the outstanding Warrants to purchase Holdings' Preferred Shares were exercised in exchange for Holdings' Preferred Shares, and all of Holdings' Preferred Shares (including the Preferred Shares issuable upon the exercise of the Warrants) were then converted into an aggregate of approximately 104.1 million of Holdings' common shares. We do not believe that this conversion affected any pre-existing rights to acquire Holdings' common shares, unless the agreement or instrument granting the right to acquire Holdings' common shares provided otherwise.
All of the outstanding Preferred Shares, immediately prior to their conversion into common shares, were held by the Equity Sponsors and their affiliates, certain members of our management, and selected distributors. In addition, affiliates of the Equity Sponsors and GarMark Partners, L.P. ("GarMark") held Warrants to purchase an aggregate of 2,040,816 of the Preferred Shares. These parties held certain rights that may have presented an actual or potential conflict of interest in connection with our proposal to convert the Preferred Shares.
Certain Equity Sponsors (and/or their affiliates) and the selected distributors holding Preferred Shares were and are parties to a shareholders' agreement pursuant to which they have certain rights to determine the composition of Holdings' Board of Directors. See "—Shareholders' Agreement."
In addition, an affiliate of Whitney, one of the Equity Sponsors, was a party to a securities purchase agreement providing that affiliate with the right to designate one observer to Holdings' Board of Directors to attend each meeting of the Board and each meeting of the committees of the Board for so long as that party holds at least $10 million of the Senior Notes (subject to certain exceptions). Holdings purchased all of the Senior Notes on March 8, 2004, the closing date of the Transactions. See "—Purchase of Senior Notes."
Purchase of Senior Notes
A portion of the proceeds from the offering of the Outstanding Notes was applied to purchase the Senior Notes at a negotiated price.
All of the Senior Notes, immediately prior to the closing of the Transactions, were held by GarMark, Whitney Private Debt Fund, L.P. ("Whitney Private Debt"), and Green River Offshore Fund Ltd. ("Green River"). Whitney Private Debt and Green River are affiliates of Whitney. GarMark purchased $23 million in principal amount of the Senior Notes and received Warrants for 1,235,231 of the Preferred Shares and Whitney Private Debt purchased $15 million in principal amount of the Senior Notes and received Warrants for 805,585 of the Preferred Shares on July 31, 2002 pursuant to a Securities Purchase Agreement among Holdings, as issuer, and GarMark and Whitney Private Debt, as purchasers. On November 27, 2002, Green River purchased $1.6 million in principal amount of the Senior Notes from GarMark and received Warrants for 85,929 of the Preferred Shares from GarMark.
The holders of the Senior Notes held certain rights that may have presented an actual or potential conflict of interest in connection with our proposal to purchase the Senior Notes. The Securities Purchase Agreement provided that each holder of $10 million or more of the Senior Notes (subject to certain exceptions) could designate one observer to Holdings' Board of Directors to attend each
105
meeting of the Board and each meeting of the committees of the Board. Each of Whitney Private Debt and GarMark held $10 million or more of the Senior Notes. In addition, certain affiliates of Whitney were and are parties to a shareholders' agreement with certain of Holdings' other shareholders pursuant to which Whitney V, L.P., an affiliate of Whitney, is permitted to nominate four individuals to Holdings' Board of Directors, and two additional nominees to Holdings' Board must be acceptable to Whitney V, L.P. and CCG Investments (BVI), L.P., an affiliate of Golden Gate Private Equity, Inc. See "—Shareholders' Agreement."
On February 3, 2004, the Board of Directors approved the offering of the Outstanding Notes and the consummation of the Transactions, subject to development of the final terms and the approval of those terms by a Special Offering Committee of the Board of Directors established to determine and approve on behalf of Holdings the final terms of the Outstanding Notes and the related Transactions. During that portion of the meeting relating to the discussion and approval of the purchase of the Senior Notes (a portion of which are owned by Whitney and its affiliates), Messrs. Peter M. Castleman, James H. Fordyce, John C. Hockin and Steven E. Rodgers, members of Holdings' Board of Directors who are also partners of Whitney and various of its affiliates, abstained from the discussion and vote. The remaining members of the Board, after considering relevant factors, determined that the purchase of our Senior Notes was desirable and in the best interests of Holdings, and approved the purchase of the Senior Notes at such price and on such terms as the Special Offering Committee deemed appropriate in connection with the sale of the Outstanding Notes.
On March 3, 2004, the Special Offering Committee approved the final terms of the Outstanding Notes and the related Transactions as set forth in this prospectus, with those of its members who are affiliated with Whitney abstaining from the discussion and vote concerning the purchase of Holdings' Senior Notes.
Certain Transactions Relating to Holdings
Transactions in securities
Selected members of our distributor organization and senior management have purchased, either from Holdings or from the Equity Sponsors, 12% Series A Cumulative Convertible Preferred Shares of Holdings. The price paid by participating members of our distributor organization and senior management to the Equity Sponsors in the August and October 31, 2002 offering was $1.76 per share. In connection with the January 31, 2003 offering to members of our President's Team by the Equity Sponsors, the price paid by distributors to the Equity Sponsors was $1.97 per share. In connection with the May 30, 2003 offering by the Equity Sponsors to members of our President's Team and by Holdings to members of our Chairman's Club, the price paid by members of our President's Team to the Equity Sponsors and by members of our Chairman's Club to Holdings was $2.21 per share. Michael O. Johnson, our Chief Executive Officer, purchased from Holdings 203,620 shares on June 24, 2003. The price paid by Mr. Johnson was the same price paid by members of our distributor organization in the May 30th offering.
In connection with a separation and general release agreement with Mr. Francis X. Tirelli effective December 24, 2002, the Equity Sponsors repurchased 284,091 Preferred Shares of Holdings held by Mr. Tirelli at a purchase price of $1.78 per share.
Shareholders' agreement
In connection with the subscription for the purchase of Holdings' shares, participating distributors and senior management are required to become party to a shareholders' agreement entered into by certain of the Equity Sponsors and Holdings (the "shareholders' agreement"). This agreement restricts the ability of the shareholders to freely transfer preferred shares and common shares held by them (other than to the transferor's family members, other shareholders and the Equity Sponsors). The
106
Equity Sponsors and their transferees may have also entered into an institutional shareholders' agreement which contains substantially similar restrictions to the shareholders' agreement relating to transfers by the Equity Sponsors and their transferees.
The Equity Sponsors also have rights of co-sale and bring-along rights on shares owned by other shareholders. The other shareholders have preemptive rights, and pro rata tag-along rights on certain sales of shares by the Equity Sponsors which will reduce the Equity Sponsors' holdings below a threshold.
If a distributor shareholder is terminated, unless such distributor sells its shares to another distributor, Holdings shall have the right to repurchase such shares. If a shareholder that is a member of senior management is terminated, first the Equity Sponsors, then Holdings shall have the right to repurchase such shares before such terminated shareholder can sell its shares to third parties. Also, under the shareholders' agreement, the shareholders will agree to vote in favor of the election of the following designees to Holdings' Board of Directors:
If the distributors own at least 11,000,000 common shares, they may designate one nominee to the Board of Directors of Holdings. If the distributors own more than 11,000,000 but less than 16,500,000 common shares of our equity, they may also select one observer to attend all meetings of our Board of Directors. If the distributors own 16,500,000 common shares or more of our equity, they may designate two nominees. The nominees of the distributors shall be elected by a plurality of the distributor shareholders.
In the event an Equity Sponsor does not have a designee serving on the Board of Directors for any reason, one person designated by such Equity Sponsor will be permitted to attend as an observer at all meetings of the Board of Directors of Holdings.
Registration rights agreement
Members of our distributor organization holding Holdings' equity securities are also party to a registration rights agreement between the Equity Sponsors and Holdings (the "Holdings registration rights agreement"). Under the Holdings registration rights agreement, the Equity Sponsors have the ability, under certain circumstances, to cause Holdings to register certain equity securities and to participate in registrations by Holdings of its equity securities. Upon an initial public offering, if the Equity Sponsors shall include their shares for registration, the other shareholders may also participate pro rata.
In addition to an initial public offering, if Holdings at any time proposes to register any of its securities under the Securities Act for sale to the public, in certain circumstances holders of preferred shares or common shares issued upon conversion of the preferred shares (including distributor shareholders) may require Holdings to include their shares in the securities to be covered by the registration statement. Such registration rights are subject to customary limitations specified in the Holdings registration rights agreement.
107
Indemnity agreement
In connection with the purchase of Preferred Shares, Holdings and WH Acquisition Corp. entered into an indemnity agreement with the Equity Sponsors pursuant to which Holdings and Herbalife (as successor-in-interest to WH Acquisition Corp.) agreed to indemnify the Equity Sponsors for losses and claims resulting from, arising out of or any way related to the Acquisition, including existing litigation. Whitney had been sued in San Francisco by Rosemont Associates and Joseph Urso for $20 million in a suit alleging breach of contract, breach of covenants of good faith and fair dealing, quantum meruit and other causes of action arising out of the sale of Herbalife to Whitney and others. This lawsuit has recently been settled for an undisclosed sum that is not material to us or our financial condition. See "—Legal Proceedings."
Agreements with the Equity Sponsors
In connection with the Acquisition, we entered into various agreements with the Equity Sponsors. Pursuant to the monitoring fee agreement entered into in connection with the Acquisition, Whitney and GGC Administration, LLC, an affiliate of CCG Investments (BVI), L.P., conduct certain activities related to such parties' and its affiliates' investments in Holdings.
In consideration of those services, Herbalife pays to Whitney and GGC Administration, LLC, quarterly, fees for monitoring services rendered (determined on an hourly basis), and such obligations are guaranteed by Holdings. Such monitoring fees are currently being paid quarterly at a rate of $5.0 million per annum, divided between Whitney and GGC Administration, LLC at a ratio of 65% to 35%, respectively. Herbalife also agreed to reimburse Whitney and GGC Administration, LLC for their reasonable out-of-pocket expenses and to pay additional transaction fees to them in the event Holdings and/or any of its subsidiaries completes add-on acquisitions, divestitures, a transaction resulting in a change of control (as defined therein) or financing involving Holdings and/or any of its subsidiaries, and that such obligations shall be guaranteed by Holdings. In fiscal 2003, Herbalife reimbursed Whitney and GGC Administration, LLC approximately $3.1 million for their reasonable out-of-pocket expenses incurred since the date of the Acquisition through the payment date. We currently anticipate that Herbalife will continue to reimburse the Equity Sponsors for approximately $2.3 million per year in reasonable, reimbursable out-of-pocket expenses.
Holdings and its subsidiaries have also agreed to provide customary joint and several indemnification to Whitney and GGC Administration, LLC. See "—Legal Proceedings" and "—Indemnity Agreement."
WH Holdings (Cayman Islands) Ltd. stock incentive plan
Holdings has established a stock incentive plan that provides for the grant of options to purchase common shares of Holdings and stock appreciation rights to employees and consultants of Herbalife. The incentive plan is administered by a committee appointed by the Board of Directors of Holdings. Upon conversion of the options into common shares of Holdings, employees of Herbalife will be required to enter into a shareholders' agreement and a registration rights agreement with Holdings. In addition, Holdings established a stock option plan that provides for the grant of options to executive officers of Herbalife. See "—Description of Benefit Plans" and "—Employment Contracts."
WH Holdings (Cayman Islands) Ltd. independent directors stock option plan
Holdings has established an independent directors stock option plan that provides for the grant of options to purchase common shares of Holdings to independent directors of Holdings. Directors who are employees of Holdings or any of its affiliates or have been designated as directors by the affiliates of Holdings or its distributors are not independent directors for purposes of director compensation. Holdings has granted options to Henry Burdick and Charles Orr under this plan.
108
DESCRIPTION OF OTHER INDEBTEDNESS
Herbalife's Senior Credit Facilities
Upon the consummation of the Acquisition, Herbalife entered into senior credit facilities with various lenders, including Whitney Private Debt Fund, L.P., and UBS AG, Stamford Branch as administrative agent. Set forth below is a summary of the terms of the senior credit facilities.
The senior credit facilities consist of (i) a senior term loan facility in the original aggregate principal amount of $180 million and (ii) a senior revolving credit facility in the aggregate principal amount of $25.0 million. The term loan will mature on June 30, 2008. The revolving credit facility is available until July 31, 2007. As of March 31, 2004, the outstanding amounts under the senior term loan facility were approximately $75.4 million. There were no amounts outstanding under the revolving credit facility as of March 31, 2004.
The senior credit facilities are guaranteed by certain of the subsidiaries of Herbalife that are also guarantors under Herbalife's senior subordinated notes. The senior credit facilities are also guaranteed by us. The obligations under the senior credit facilities are secured by (i) first priority pledges of (A) all of the stock of the guarantors, and (B) with limited exceptions, 65% of the equity interests of the foreign subsidiaries of Herbalife that are not guarantors, and (ii) security interests in and liens on all accounts receivable, inventory and other property and assets of ours and the guarantors.
All amounts outstanding under Herbalife's senior credit facilities bear interest, at its option, subject to certain limitations, as follows: (i) with respect to our senior revolving credit facility: at the base rate plus 2.75% per annum or at the reserve adjusted LIBOR Rate plus 3.75% per annum; and (ii) with respect to amounts outstanding under our senior term loan facility: at the base rate plus 3.00% per annum or at the reserve adjusted LIBOR Rate plus 4.00% per annum.
The applicable margin for the revolving loans under the senior credit facilities is subject to adjustment based upon the consolidated total leverage ratio of Herbalife. The base rate is a fluctuating interest rate equal to the higher of (a) UBS' prime rate or (b) the Federal Funds Effective Rate plus 0.5%. Herbalife also pays customary administration fees and expenses and commitment fees of 0.50% per annum on the unused portion of the revolving credit facility.
Herbalife may prepay borrowings under the senior credit facilities in whole or in part, in minimum amounts and subject to certain other conditions set forth in the credit agreement. Subject to specified exceptions, Herbalife is currently required to make mandatory prepayments to the lenders from certain asset sales, from proceeds of new debt or equity issuances, from insurance proceeds and from excess cash flow.
In addition, the senior credit facilities contain various covenants, including, without limitation, financial covenants as well as restrictions on Herbalife's ability to:
The senior credit facilities also include customary indemnities and events of default, including a change of control, as defined in the senior credit facilities.
109
We amended and restated Herbalife's senior credit facility in order to (i) permit the issuance of the Outstanding Notes and the New Notes offered hereby and the use of proceeds therefrom, (ii) decrease the applicable margins on the interest rates applicable to loans under the senior credit facility, (iii) waive the excess cash flow prepayment required for the 2003 fiscal year, (iv) permit the funding of interest and certain tax payments on the Outstanding Notes and the New Notes offered hereby so long as no default exists or would result therefrom, (v) permit certain sales by Herbalife to guarantors of certain assets relating to foreign operations and equity interests in foreign subsidiaries and (vi) permit certain additional flexibility in adjustments to the capital structure of Holdings and its subsidiaries.
Herbalife's Senior Subordinated Notes
On June 27, 2002, WH Acquisition Corp. issued $165.0 million aggregate principal amount of 113/4% senior subordinated notes due 2010. The notes were initially purchased by UBS Warburg, LLC. The notes were resold to various qualified institutional buyers and non-U.S. persons pursuant to Rule 144A and Rule 903 or Rule 904, respectively, under the Securities Act of 1933. Upon consummation of the merger of WH Acquisition Corp. with and into Herbalife on July 31, 2002, Herbalife assumed the obligations of WH Acquisition Corp. under the Notes. In September, 2003 Herbalife repurchased $5 million aggregate principal amount of its senior subordinated Notes.
Interest on the notes is payable semi-annually on January 15 and July 15 of each year, and the notes mature on January 15, 2010. The notes are guaranteed by all of the existing subsidiaries of WH Intermediate Holdings, including the subsidiaries of Herbalife, that are guarantors under Herbalife's senior credit facilities, and, under the circumstances specified in the indenture future subsidiaries will also be required to guarantee the notes. The notes are unsecured and junior to Herbalife's obligations under its senior credit facilities and any future senior indebtedness.
Herbalife has the option to redeem the notes, in whole or in part, at any time on or after July 15, 2006 at redemption prices declining ratably from 105.875% of their principal amount on July 15, 2006 to 100% of their principal amount on or after July 15, 2008, plus accrued interest. At any time on or prior to July 15, 2005, Herbalife may also redeem up to 35% of the aggregate principal amount of the notes at a redemption price of 111.750% of their principal amount, plus accrued interest, upon certain offerings of securities of WH Intermediate Holdings. Upon a change of control, as defined in the indenture pursuant to which the notes were issued, Herbalife is required to offer to purchase the notes at a purchase price equal to 101% of their principal amount, plus accrued interest.
In addition, under certain conditions, if WH Intermediate Holdings has excess cash flow for any fiscal year, then Herbalife is required to use such proceeds to prepay, repay, redeem or purchase senior indebtedness of Herbalife or to make an offer to the holders of the notes and such other indebtedness ranking on a parity with the notes at a purchase price in cash equal to 100% of the principal amount (or accreted value in the case of Indebtedness issued with original issue discount) of the notes or such other indebtedness to be purchased, together with accrued and unpaid interest and liquidated damages, if any, thereon to the repurchase date.
The indenture governing the notes contains covenants that limits WH Intermediate Holdings and Herbalife, including certain of their respective subsidiaries', ability to, among other things:
110
On March 8, 2004, WH Holdings (Cayman Islands) Ltd., a Cayman Islands exempted limited liability company ("Holdings" or sometimes referred to herein as the "Company"), and WH Capital Corporation, a Nevada corporation ("Capital," and, together with Holdings, the "Issuers"), as Issuers, issued the Outstanding Notes (the "Outstanding Notes"). The Outstanding Notes were issued pursuant to an indenture dated March 8, 2004 (the "Indenture") among the Issuers and The Bank of New York, as trustee (the "Trustee"). The Indenture will also govern the terms and conditions of the New Notes, which we will issue in exchange for the Outstanding Notes under the Indenture.
The terms of the New Notes and the Outstanding Notes are identical in all material respects, except the New Notes:
Any Outstanding Notes that remain outstanding after the exchange offer, together with the New Notes issued in the exchange offer, will be treated as a single class of securities under the indenture for voting purposes.
The following summaries of certain provisions of the Indenture and the Registration Rights Agreement dated March 8, 2004 (the "Registration Rights Agreement") among the Issuers and the Initial Purchaser are summaries only, do not purport to be complete and are qualified in their entirety by reference to all of the provisions of the Indenture and the Registration Rights Agreement, as the case may be.
You can find the definitions of certain capitalized terms in this section under the subheading "—Certain Definitions." For purposes of this section, references to the "Issuers" or "we," "our," or "us" include only Holdings and Capital and their respective successors in accordance with the terms of the Indenture and not our subsidiaries and references to the "Notes" include both the New Notes and the Outstanding Notes (to the extent not exchanged for New Notes).
The terms of the Notes include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act of 1939, as amended (the "TIA"). The Notes are subject to all such terms, and the holders of the Notes (the "Holders") are referred to the Indenture and the TIA for a statement thereof. A copy of the form of the Indenture is available from the Trustee upon request.
The following description is a summary of the material provisions of the Indenture. It does not restate the Indenture in its entirety. We urge you to read the Indenture because the Indenture, and not this description, defines your rights as a Holder.
Brief Description of the Notes
The Notes
The Notes are:
111
The Notes will be issued in fully registered form only, without coupons, in denominations of $1,000 and integral multiples thereof.
The term "Subsidiaries" as used in this Description of Notes does not include Unrestricted Subsidiaries. As of the Issue Date, none of our subsidiaries were Unrestricted Subsidiaries.
Principal, Maturity and Interest; Additional Notes
On the Issue Date, we initially issued Notes with a maximum aggregate principal amount of $275.0 million. The Indenture provides, in addition to the Notes being issued on the Issue Date, for the issuance of additional Notes having identical terms and conditions to the Notes offered hereby (the "Additional Notes"), subject to compliance with the terms of the Indenture, including the covenant "Limitation on Incurrence of Additional Indebtedness and Disqualified Capital Stock." Any such Additional Notes would be issued on the same terms as the Notes offered hereby and would constitute part of the same series of securities as the Notes and would vote together as one series on all matters. All references to Notes herein includes the Additional Notes, except as stated otherwise.
The Notes will mature on April 1, 2011. The Notes will bear interest at 91/2% per annum from March 8, 2004 or from the most recent date to which interest has been paid or provided for (the "Interest Payment Date"), payable semi-annually in arrear on April 1 and October 1 of each year, commencing October 1, 2004, to the Persons in whose names such Notes are registered at the close of business on March 15 or September 15 immediately preceding such Interest Payment Date. Interest will be calculated on the basis of a 360-day year consisting of twelve 30-day months.
Methods of Receiving Payments on the Notes
Principal of, premium, if any, and interest (and Liquidated Damages, if any) on the Notes will be payable, and the Notes may be presented for registration of transfer or exchange, at our office or agency maintained for such purpose, which office or agency shall be maintained in the Borough of Manhattan, The City of New York. Except as set forth below, at our option, payment of interest may be made by check mailed to the Holders at the addresses set forth upon our registry books. (See "Form and Transfer of the Notes—Same Day Settlement and Payment"). No service charge will be made for any registration of transfer or exchange of the Notes, but we may require payment of a sum sufficient to cover any tax or other governmental charge payable in connection therewith. Until otherwise designated by us, our office or agency will be the corporate trust office of the Trustee presently located at the office of the Trustee in the Borough of Manhattan, The City of New York.
Subordination to Credit Agreement Indebtedness
The Notes will be our general, unsecured obligations, contractually subordinated in right of payment only to Indebtedness outstanding under the Credit Agreement. This effectively means that holders of Indebtedness outstanding under the Credit Agreement must be paid in full before any amounts are paid to the Holders in the event a bankruptcy or insolvency proceeding is commenced by or against us and that holders of Indebtedness can block payments to the Holders in the event of a default by us on such Indebtedness, all as more fully described below.
At March 31, 2004, we had outstanding our secured guaranty of an aggregate of approximately $75.4 million of Indebtedness outstanding under the Credit Agreement, and no other Indebtedness other than the Notes.
The rights of Holders will be effectively subordinated to all existing and future indebtedness and preferred stock of our subsidiaries.
112
We may not make payment (by setoff or otherwise), as applicable, on account of any Obligation in respect of the Notes, including the principal of, premium, if any, or interest on the Notes or Liquidated Damages, or on account of the redemption provisions of the Notes (including any repurchases of Notes), for cash or property (other than payments made with Junior Securities):
Upon (1) the happening of an event of default, other than a Payment Default, that permits the holders of Indebtedness outstanding under the Credit Agreement to declare such Indebtedness to be due and payable and (2) written notice of such event of default given to us by the representative under the Credit Agreement (a "Payment Blockage Notice"), then, unless and until such event of default has been cured or waived, no payment (by setoff or otherwise) may be made by us or on our behalf on account of any Obligation in respect of the Notes, including the principal of, premium, if any, or interest on the Notes, (including any repurchases of any of the Notes), or on account of the redemption provisions of the Notes (or Liquidated Damages), in any such case, other than payments made with Junior Securities. Notwithstanding the foregoing, unless the Indebtedness outstanding under the Credit Agreement has been declared due and payable within 179 days after the Payment Blockage Notice is delivered as set forth above (the "Payment Blockage Period") (and such declaration has not been rescinded or waived), at the end of the Payment Blockage Period, we shall be required to pay all sums not previously paid to the Holders during the Payment Blockage Period due to the foregoing prohibitions and to resume all other payments as and when due on the Notes.
Any number of Payment Blockage Notices may be given; provided, however, that:
Upon any distribution of our assets upon any dissolution, winding up, total or partial liquidation or reorganization of us, whether voluntary or involuntary, in bankruptcy, insolvency, receivership or a similar proceeding or upon assignment for the benefit of creditors or any marshaling of assets or liabilities:
113
In the event that, notwithstanding the foregoing, any payment or distribution of our assets (other than payments made with Junior Securities) shall be received by the Trustee or the Holders at a time when such payment or distribution is prohibited by the foregoing provisions, such payment or distribution shall be held in trust for the benefit of the holders of the Indebtedness outstanding under the Credit Agreement, and shall be paid or delivered by the Trustee or such Holders, as the case may be, to the holders of the Indebtedness outstanding under the Credit Agreement remaining unpaid or unprovided for or to their representative or representatives, or to the trustee or trustees under any indenture pursuant to which any instruments evidencing any of such Indebtedness may have been issued, ratably according to the aggregate principal amounts remaining unpaid on account of such Indebtedness held or represented by each, for application to the payment of all such Indebtedness remaining unpaid, to the extent necessary to pay or to provide for the payment of all such Indebtedness in full in cash or Cash Equivalents after giving effect to any concurrent payment or distribution to the holders of such Indebtedness.
No provision contained in the Indenture or the Notes will affect our obligation, which is absolute and unconditional, to pay, when due, principal of, premium, if any, and interest or if applicable, Liquidated Damages on the Notes. The subordination provisions of the Indenture and the Notes will not prevent the occurrence of any Default or Event of Default under the Indenture or limit the rights of the Trustee or any Holder to pursue any other rights or remedies with respect to the Notes.
As a result of these subordination provisions, in the event of the liquidation, bankruptcy, reorganization, insolvency, receivership or similar proceeding or an assignment for the benefit of our creditors or a marshaling of our assets and liabilities, the Holders may receive ratably less than other creditors.
Optional Redemption
At any time prior to April 1, 2008, we may redeem the Notes for cash, in whole or in part, from time to time, upon not less than 30 nor more than 60 days' notice to each Holder of the Notes to be redeemed at a redemption price equal to 100% of the principal amount of the Notes to be redeemed plus the Applicable Premium as of, and accrued and unpaid interest, including Liquidated Damages, if any, to the date of the redemption (the date of any such redemption prior to April 1, 2008, an "Early Redemption Date").
At any time on or after April 1, 2008, we may redeem the Notes for cash, in whole or in part, upon not less than 30 days nor more than 60 days notice to each Holder of Notes to be redeemed, at the following redemption prices (expressed as percentages of the principal amount) if redeemed during the 12-month period commencing April 1 of the years indicated below, in each case together with accrued and unpaid interest and Liquidated Damages, if any, thereon to the date of redemption of the
114
Notes (the date of any such redemption, together with any Early Redemption Date and any Equity Proceeds Redemption Date referred to below, a "Redemption Date"):
| Year |
Percentage |
||
|---|---|---|---|
| 2008 | 104.750 | % | |
| 2009 | 102.375 | % | |
| 2010 and thereafter | 100.000 | % |
At any time or from time to time on or prior to April 1, 2007, upon one or more Qualified Equity Offerings, up to 40% of the aggregate principal amount of the Notes issued pursuant to the Indenture may be redeemed at our option within 90 days of such Qualified Equity Offering, on not less than 30 days, but not more than 60 days, notice to each Holder of the Notes to be redeemed, with cash received by us from the Net Cash Proceeds of such Qualified Equity Offering, at a redemption price equal to 109.50% of principal, together with accrued and unpaid interest and Liquidated Damages, if any, thereon to the date of redemption of the Notes (any such date, an "Equity Proceeds Redemption Date"); provided, however, that immediately following such redemption not less than 60% of the aggregate principal amount of the Notes originally issued pursuant to the Indenture on the Issue Date remain outstanding.
If a Redemption Date hereunder is on or after an interest record date ("Record Date") on which the Holders of record have a right to receive the corresponding Interest due and Liquidated Damages, if any, and on or before the associated Interest Payment Date, any accrued and unpaid interest and Liquidated Damages, if any, due on such Interest Payment Date will be paid to the Person in whose name a Note is registered at the close of business on such Record Date.
Selection and Notice
In the case of a partial redemption, the Trustee shall select the Notes or portions thereof for redemption on a pro rata basis, by lot or in such other manner it deems appropriate and fair. The Notes may be redeemed in part in multiples of $1,000 only.
Notice of any redemption will be sent, by first class mail, at least 30 days and not more than 60 days prior to the date fixed for redemption to the Holder of each Note to be redeemed to such Holder's last address as then shown upon the registry books of our registrar. Any notice which relates to a Note to be redeemed in part only must state the portion of the principal amount equal to the unredeemed portion thereof and must state that on and after the date of redemption, upon surrender of such Note, a New Note or Notes in a principal amount equal to the unredeemed portion thereof will be issued. On and after the date of redemption, interest will cease to accrue on the Notes or portions thereof called for redemption, unless we default in the payment thereof.
Repurchase at the Option of the Holders
Repurchase of Notes at the option of the holder upon a change of control
The Indenture provides that in the event that a Change of Control has occurred, each Holder will have the right, at such Holder's option, pursuant to an offer (subject only to conditions required by applicable law, if any) by us (the "Change of Control Offer"), to require us to repurchase all or any part of such Holder's Notes (provided, that the principal amount of such Notes must be $1,000 or an integral multiple thereof) on a date (the "Change of Control Repurchase Date") that is no later than 45 Business Days after the occurrence of such Change of Control, at a cash price equal to 101% of the principal amount thereof (the "Change of Control Purchase Price"), together with accrued and unpaid interest and Liquidated Damages, if any, to the Change of Control Repurchase Date.
The Change of Control Offer shall be made within 20 Business Days following a Change of Control and shall remain open for 20 Business Days following its commencement or such other period
115
as may be required by applicable law (the "Change of Control Offer Period"). Upon expiration of the Change of Control Offer Period, we shall promptly purchase all Notes properly tendered in response to the Change of Control Offer.
As used herein, a "Change of Control" means:
provided, however that any consolidation, merger, sale, lease, conveyance or transfer of assets (including upon a dissolution or liquidation) pursuant to and in accordance with the covenant "Limitation on Merger, Sale or Consolidation" shall not constitute a "Change on Control" under clauses (3), (4), (5) or (6) above.
As used in this covenant, "person" (including any group that is deemed to be a "person") has the meaning given by Sections 13(d) of the Exchange Act, whether or not applicable.
Notwithstanding the foregoing, we are not required to make a Change of Control Offer upon a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by us, including any requirements to repay in full all Indebtedness outstanding under the Credit Agreement as set forth in the following paragraph of this Section, and purchases all Notes validly tendered and not withdrawn under such Change of Control Offer.
116
The Indenture provides that, prior to the commencement of a Change of Control Offer, but in any event within 30 days following any Change of Control, we will:
Our failure to comply with the preceding sentence shall constitute an Event of Default described in clause (3) under "Events of Default" below.
On or before the Change of Control Repurchase Date, we will:
The Paying Agent promptly will pay the Holders so accepted an amount equal to the Change of Control Purchase Price (together with accrued and unpaid interest and Liquidated Damages, if any, to the Change of Control Repurchase Date) and the Trustee promptly will authenticate and deliver to such Holders a New Note equal in principal amount to any unpurchased portion of the Note surrendered. Any Notes not so accepted will be delivered promptly by us to the Holder thereof. We publicly will announce the results of the Change of Control Offer on or as soon as practicable after the Change of Control Repurchase Date.
The Change of Control purchase feature of the Notes may make more difficult or discourage a takeover of us, and, thus, the removal of incumbent management.
The phrase "all or substantially all" of a Person's assets will likely be interpreted under applicable state law and will be dependent upon particular facts and circumstances. As a result, there may be a degree of uncertainty in ascertaining whether a sale or transfer of "all or substantially all" of our assets has occurred. In addition, no assurances can be given that we will be able to acquire the Notes tendered upon the occurrence of a Change of Control.
Any Change of Control Offer will be made in compliance with all applicable laws, rules and regulations, including, if applicable, Regulation 14E under the Exchange Act and the rules thereunder and all other applicable Federal and state securities laws. To the extent that the provisions of any securities laws or regulations conflict with the provisions of this covenant, our compliance with such laws and regulations shall not in and of itself cause a breach of our obligations under such covenant.
If the Change of Control Repurchase Date hereunder is on or after an interest payment Record Date and on or before the associated Interest Payment Date, any accrued and unpaid interest (and Liquidated Damages, if any) due on such Interest Payment Date will be paid to the Person in whose name a Note is registered at the close of business on such Record Date.
Sale of assets and subsidiary stock
The Indenture provides that we will not, and we will not permit any of our Subsidiaries to, in one or a series of related transactions, sell, transfer, assign or otherwise dispose of, directly or indirectly, any of their property, business or assets, including by merger or consolidation (in the case of a Subsidiary of the Company), and including any sale or other transfer or issuance of any Equity Interests of any Subsidiary of the Company, whether by the Company or through the issuance, sale or
117
transfer of Equity Interests by a Subsidiary of the Company and including any sale and leaseback transaction (any of the foregoing, an "Asset Sale"), unless:
For purposes of (1) above, total consideration received means the total consideration received for such Asset Sales minus the amount of (a) Indebtedness assumed by a transferee in an Asset Sale and (b) property that within 30 days of such Asset Sale is converted into cash or Cash Equivalents; provided, that such cash and Cash Equivalents shall be treated as Net Cash Proceeds attributable to the original Asset Sale for which such property was received.
The Indenture provides that within 360 days following such Asset Sale, the Net Cash Proceeds therefrom (the "Asset Sale Amount") are:
Pending the final application of any Net Cash Proceeds, the Company may temporarily reduce revolving credit borrowings or otherwise invest the Net Cash Proceeds in any manner that is not prohibited by the Indenture.
The accumulated Net Cash Proceeds from Asset Sales not applied as set forth in (a), (b) or (c) of the preceding paragraph shall constitute Excess Proceeds. Within 30 days after the date that the amount of Excess Proceeds exceeds $15.0 million, the Company shall apply the Excess Proceeds (the "Asset Sale Offer Amount") to the repurchase of the Notes and such other Indebtedness ranking on a parity with the Notes and with similar provisions requiring us to make an offer to purchase such Indebtedness with the proceeds from such Asset Sale pursuant to a cash offer (subject only to conditions required by applicable law, if any) (pro rata in proportion to the respective principal amounts (or accreted values in the case of Indebtedness issued with an original issue discount) of the Notes and such other Indebtedness then outstanding) (the "Asset Sale Offer") at a purchase price of 100% of the principal amount (or accreted value in the case of Indebtedness issued with an original
118
issue discount) (the "Asset Sale Offer Price") together with accrued and unpaid interest and Liquidated Damages, if any, to the date of payment. Each Asset Sale Offer shall remain open for 20 Business Days following its commencement (the "Asset Sale Offer Period").
Upon expiration of the Asset Sale Offer Period, we shall apply the Asset Sale Offer Amount plus an amount equal to accrued and unpaid interest and Liquidated Damages, if any, to the purchase of all Indebtedness properly tendered in accordance with the provisions hereof (on a pro rata basis if the Asset Sale Offer Amount is insufficient to purchase all Indebtedness so tendered) at the Asset Sale Offer Price (together with accrued interest and Liquidated Damages, if any, to the redemption date). To the extent that the aggregate amount of Notes and such other pari passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Asset Sale Offer Amount, we may use any remaining Net Cash Proceeds in any manner not otherwise prohibited by the Indenture and following the consummation of each Asset Sale Offer the Excess Proceeds amount shall be reset to zero.
Notwithstanding, and without complying with, the provisions of this covenant:
Any Asset Sale Offer shall be made in compliance with all applicable laws, rules, and regulations, including, if applicable, Regulation 14E of the Exchange Act and the rules and regulations thereunder and all other applicable Federal and state securities laws. To the extent that the provisions of any securities laws or regulations conflict with the provisions of this paragraph, our compliance or the compliance of any of our subsidiaries with such laws and regulations shall not in and of itself cause a breach of our obligations under such covenant.
If the payment date in connection with an Asset Sale Offer hereunder is on or after an interest payment Record Date and on or before the associated Interest Payment Date, any accrued and unpaid interest (and Liquidated Damages, if any) due on such Interest Payment Date will be paid to the Person in whose name a Note is registered at the close of business on such Record Date.
119
The Indenture contains certain covenants that, among other things, restrict our ability and the ability of our Subsidiaries to borrow money, grant liens, pay dividends on or repurchase capital stock, make investments and sell assets or enter into mergers or consolidations. The following summary of certain covenants of the Indenture are summaries only, do not purport to be complete and are qualified in their entirety by reference to all of the provisions of the Indenture. We urge you to read the Indenture because the Indenture, and not this description, details your rights as a holder of the Notes.
Limitation on incurrence of additional indebtedness and disqualified capital stock
The Indenture provides that, except as set forth in this covenant, we will not and we will not permit any of our Subsidiaries to, directly or indirectly, issue, assume, guaranty, incur, become directly or indirectly liable with respect to (including as a result of an Acquisition), or otherwise become responsible for, contingently or otherwise (individually and collectively, to "incur" or, as appropriate, an "incurrence"), any Indebtedness (including Disqualified Capital Stock and Acquired Indebtedness), other than Permitted Indebtedness.
Notwithstanding the foregoing if:
then we and our Subsidiaries may incur such Indebtedness (including Disqualified Capital Stock).
In addition, the foregoing limitations of the first paragraph of this covenant will not prohibit:
120
the definition thereof (plus any Refinancing Indebtedness incurred to retire, defease, refinance, replace or refund such Indebtedness) of up to $205.0 million (or the equivalent thereof at the time of incurrence in the applicable foreign currency), minus the amount of any such Indebtedness (1) retired with the Net Cash Proceeds from any Asset Sale applied to permanently reduce the outstanding amounts or the commitments with respect to such Indebtedness pursuant to clause (b) of the second paragraph of the covenant "Sale of Assets and Subsidiary Stock," (2) assumed by a transferee in an Asset Sale and (3) the aggregate amount of all mandatory principal payments and prepayments in respect of term loans thereunder (excluding any such payments to the extent refinanced at the time of payment under a replacement or refinancing thereof) actually made; provided, that, this clause (3) shall not reduce the aggregate amount of Indebtedness available to be incurred and outstanding by the Company and its Subsidiaries pursuant to this clause (c) below $35.0 million.
In addition, the Company will not permit WH Intermediate, Luxembourg Holdings, Luxembourg Intermediate Holdings or Herbalife to refinance, redeem, repurchase or repay the Herbalife Notes or issue additional Indebtedness (other than Senior Indebtedness) unless WH Intermediate, Luxembourg Holdings, Luxembourg Intermediate Holdings, Herbalife and all of their respective subsidiaries that have guaranteed such Indebtedness fully and unconditionally guarantee the Notes; provided that such guarantee shall be subordinated to Senior Indebtedness of WH Intermediate, Luxembourg Holdings, Luxembourg Intermediate Holdings, Herbalife and such subsidiaries in substantially the same manner that the Herbalife Notes and the related guarantees are subordinated to Senior Indebtedness; provided, further, that the obligation to execute any such guarantee shall not apply if and for so long as such guarantee is restricted or prohibited by the terms of any Senior Indebtedness of WH Intermediate, Luxembourg Holdings, Luxembourg Intermediate Holdings or Herbalife. The Company will not permit WH Intermediate, Luxembourg Holdings, Luxembourg Intermediate Holdings or Herbalife to issue any Indebtedness (other than Credit Agreement) after the Issue Date that restricts or prohibits the guarantee of the Notes required by the prior sentence. The terms of Herbalife's amended and restated credit agreement would currently restrict such guarantee.
Indebtedness (including Disqualified Capital Stock) of any Person which is outstanding at the time such Person becomes a Subsidiary of the Company (including upon designation of any subsidiary or other Person as a Subsidiary of the Company) or is merged with or into or consolidated with the Company or a Subsidiary of the Company shall be deemed to have been incurred at the time such Person becomes or is designated a Subsidiary of the Company or is merged with or into or consolidated with the Company or a Subsidiary of the Company.
Notwithstanding any other provision of this covenant, but only to avoid duplication, a guarantee of Indebtedness of the Company or any Subsidiary of the Company incurred in accordance with the terms of the Indenture (other than Indebtedness incurred pursuant to clause (a) hereof) issued at the time such Indebtedness was incurred or if later at the time the guarantor thereof became a Subsidiary of the Company will not constitute a separate incurrence, or amount outstanding, of Indebtedness. For purposes of determining compliance with this covenant, in the event that an item of Indebtedness is entitled to be incurred pursuant to this covenant or one or more clauses of the definition of Permitted Indebtedness, the Company shall, in its sole discretion, classify (or later reclassify in whole or in part, in its sole discretion) such item of Indebtedness in any manner that complies with this covenant.
Limitation on restricted payments
The Indenture provides that we will not, and we will not permit any Subsidiary of the Company to, directly or indirectly, make any Restricted Payment if, after giving effect to such Restricted Payment on a pro forma basis:
121
The foregoing clauses (2) and (3) of the immediately preceding paragraph, however, will not prohibit:
122
The foregoing clauses (1), (2) and (3) of the preceding paragraph will not prohibit:
The full amount of any Restricted Payment made pursuant to the foregoing clauses (A), (D), and (F) (but not pursuant to clauses (B), (C) or (E)) of the immediately preceding sentence, however, will be counted as Restricted Payments made for purposes of the calculation of the aggregate amount of Restricted Payments available to be made referred to in clause (3) of the first paragraph under the heading "—Limitation on Restricted Payments;" provided, however, that if there is a Final Determination in respect of any particular Tax Determination Year for which a Tax Amounts Payment has been disbursed pursuant to the foregoing clause (B), the Final Determination Amount related thereto (other than interest and penalties) will be counted as a Restricted Payment made for purposes of the calculation of such aggregate Restricted Payments from and after the date such Final Determination is made.
For purposes of this covenant, the amount of any Restricted Payment made or returned, if other than in cash, shall be the fair market value thereof, as determined in the good faith reasonable judgment of our Board of Directors, at the time made or returned, as applicable. Additionally, within 5 days of each Restricted Payment in excess of $250,000 that is not a Restricted Investment, we shall deliver an Officers' Certificate to the Trustee describing in reasonable detail the nature of such Restricted Payment, stating the amount of such Restricted Payment, stating in reasonable detail the provisions of the Indenture pursuant to which such Restricted Payment was made and certifying that such Restricted Payment was made in compliance with the terms of the Indenture.
Limitation on dividends and other payment restrictions affecting subsidiaries
The Indenture provides that we will not and we will not permit any Subsidiary of the Company to, directly or indirectly, create, assume or suffer to exist any consensual restriction on the ability of any Subsidiary of the Company to pay dividends or make other distributions to or on behalf of, or to pay any obligation to or on behalf of, or otherwise to transfer assets or property to or on behalf of, or make or pay loans or advances to or on behalf of, the Company or any Subsidiary of the Company, except:
123
of acquisition, were not put in place in connection with or in anticipation of such acquisition and are not applicable to any Person, other than the Person acquired, or to any property, asset or business, other than the property, assets and business so acquired,
Notwithstanding the foregoing, (a) customary provisions restricting subletting or assignment of any lease entered into in the ordinary course of business, consistent with industry practice shall not be prohibited by this covenant and (b) any asset subject to a Lien which is not prohibited to exist with respect to such asset pursuant to the terms of the Indenture may be subject to customary restrictions on the transfer or disposition thereof pursuant to such Lien.
Limitation on liens securing indebtedness
We will not create, incur, assume or suffer to exist any Lien of any kind, other than Permitted Liens, upon any of our assets now owned or acquired on or after the date of the Indenture securing any of our Indebtedness, unless we provide that the Notes are equally and ratably so secured; provided that if such Indebtedness is Subordinated Indebtedness, the Lien securing such Subordinated Indebtedness shall be contractually subordinate and junior to the Lien securing the Notes with the same relative priority as such Subordinated Indebtedness shall have with respect to the Notes.
Limitation on transactions with affiliates
The Indenture provides that we will not and will not let any Subsidiary of the Company, on or after the Issue Date, enter into or suffer to exist any contract, agreement, arrangement or transaction with any Affiliate (an "Affiliate Transaction"), or any series of related Affiliate Transactions, (other than Exempted Affiliate Transactions), (1) unless it is determined that the terms of such Affiliate Transaction are fair and reasonable to us, and no less favorable to us than could have been obtained in an arm's length transaction with a non-Affiliate, and (2) if involving consideration to either party in excess of $5.0 million, unless such Affiliate Transaction(s) has been approved by a majority of the members of our Board of Directors that are disinterested in such transaction, if there are any directors who are so disinterested, and (3) if involving consideration to either party in excess of $10.0 million, or
124
$7.5 million if there are no disinterested directors for such transaction, unless, in addition we, prior to the consummation thereof, obtain a written favorable opinion as to the fairness of such transaction to us from a financial point of view from an independent investment banking firm of national reputation in the United States or, if pertaining to a matter for which such investment banking firms do not customarily render such opinions, an appraisal or valuation firm of national reputation in the United States. Within 5 days of any Affiliate Transaction(s) involving consideration to either party of $5.0 million or more (other than Exempted Affiliate Transactions), the Company shall deliver to the Trustee an Officers' Certificate addressed to the Trustee certifying that such Affiliate Transaction complied with clause (1), (2), and (3), as applicable.
Limitation on merger, sale or consolidation of the company
The Indenture provides that we will not consolidate with or merge with or into another Person, or, directly or indirectly, sell, lease, convey or transfer (including by means of a dissolution or liquidation) all or substantially all of the Company's assets (such amounts to be computed on a consolidated basis), whether in a single transaction or a series of related transactions, to another Person or group of affiliated Persons or adopt a plan of liquidation, unless:
Upon any consolidation, merger, sale, conveyance or transfer (including by means of a dissolution or liquidation) in accordance with the foregoing, the successor corporation formed by such consolidation or into which the Company is merged or the transferee corporation shall succeed to and (except in the case of a lease or a sale of less than all of the Company's assets) be substituted for, and may exercise every right and power of, the Company under the Indenture with the same effect as if such successor or transferee corporation had been named therein as the Company, and (except in the case of a lease or a sale of less than all of the Company's assets) the Company shall be released from the obligations under the Notes and the Indenture except with respect to any obligations that arise from, or are related to, such transaction.
For purposes of the foregoing, the transfer (by lease, assignment, sale or otherwise) of all or substantially all of the properties and assets of one or more Subsidiaries, the Company's interest in which constitutes all or substantially all of the Company's properties and assets, shall be deemed to be the transfer of all or substantially all of the Company's properties and assets.
125
Limitation on lines of business
The Indenture provides that neither the Company nor any Subsidiary of the Company will directly or indirectly engage to any substantial extent in any line or lines of business activity other than that which, in the reasonable good faith judgment of our Board of Directors, is a Related Business.
Limitation on guarantees
If any Subsidiary of the Company guarantees any of the Company's Indebtedness (other than Indebtedness outstanding under the Credit Agreement), or suffers to exist any Lien on any of such Subsidiary's assets to secure any Indebtedness of the Company (other than Indebtedness in respect of the Company's guaranty of Indebtedness outstanding under the Credit Agreement), then such Subsidiary must cause the Notes to be guaranteed or secured, as the case may be, on an equal and ratable basis.
Limitation on mergers, consolidations, liquidations and dissolutions of Guarantors; Release of Guarantors
The Indenture provides that no Guarantor will consolidate or merge with or into (whether or not such Guarantor is the surviving Person) another Person unless: (1) subject to the provisions of the following paragraph and the other provisions of the Indenture, the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) assumes all the obligations of such Guarantor pursuant to a supplemental indenture in form reasonably satisfactory to the Trustee, pursuant to which such Person shall guarantee all of such Guarantor's obligations under such Guarantor's guarantee of the Notes to the same extent that such Guarantor had guaranteed the Notes; and (2) immediately before and immediately after giving effect to such transaction on a pro forma basis, no Default or Event of Default shall have occurred or be continuing. The provisions of the covenant shall not apply to the merger of any Guarantors with and into each other or with or into the Company.
Upon the sale or disposition (including by merger or stock purchase) of a Guarantor (as an entirety) to an entity which is not and is not required to become a Guarantor, or the designation of a Subsidiary to become an Unrestricted Subsidiary, which transaction is otherwise in compliance with the Indenture (including, without limitation, the provisions of the covenant "Limitations of Sale of Assets and Subsidiary Stock"), such Guarantor will be deemed released from its obligations under its guarantee of the Notes; provided, however, that any such termination shall occur only to the extent that all obligations of such Guarantor under all of its guarantees of, and under all of its pledges of assets or other security interest which secure, any Indebtedness of the Company or any Indebtedness of any other Subsidiary of the Company shall also terminate upon such release, sale or transfer and none of its Equity Interests are pledged for the benefit of any holder of any Indebtedness of the Company or any Indebtedness of any Subsidiary of the Company.
Limitation on status as investment company
The Indenture prohibits the Company and each of the Subsidiaries of the Company from being required to register as an "investment company" (as that term is defined in the Investment Company Act of 1940, as amended), or from otherwise becoming subject to regulation under the Investment Company Act.
Limitation on activities of Capital
The Indenture provides that Capital will not hold any material assets (other than Capital Stock of any Subsidiary of the Company or of another co-obligor of the Notes) or engage in any business activities (other than holding any such Capital Stock); provided, that Capital may be a co-obligor of the Notes or any other Indebtedness of the Company incurred in accordance with the covenant described
126
above under the caption "—Limitation on Incurrence of Indebtedness." Capital may, as necessary, engage in any activities directly related or necessary in connection therewith.
Reports
The Indenture provides that, whether or not the Issuers are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act, the Issuers will deliver to the Trustee and, to each Holder within 5 days after the Issuers are or would have been (if the Issuers were subject to such reporting obligations) required to file such with the Commission, annual and quarterly financial statements substantially equivalent to financial statements that would have been included in reports filed with the Commission, if the Issuers were subject to the requirements of Section 13 or 15(d) of the Exchange Act, including, with respect to annual information only, a report thereon by our certified independent public accountants as such would be required in such reports to the Commission, and, in each case, together with a management's discussion and analysis of financial condition and results of operations which would be so required and, from and after consummation of the Exchange Offer, unless the Commission will not accept such reports, file with the Commission the annual, quarterly and other reports which it is or would have been required to file with the Commission.
Additional Amounts
All amounts paid or credited by the Company under or with respect to the Notes will be made free and clear of and without withholding or deduction for or on account of any present or future tax, duty, levy, impost, assessment, or other governmental charge imposed or levied by or on behalf of the Government of the Cayman Islands (or any successor jurisdiction, in the case of any successor corporation to the Company in accordance with the covenant "Limitation on merger, sale or consolidation of the company") or of any political subdivision thereof or by any authority or agency therein or thereof having power to tax (hereinafter "Taxes"), unless the Company is required to withhold or deduct Taxes by law or by the interpretation or administration thereof by the relevant government authority or agency. If the Company is so required to withhold or deduct any amount for or on account of Taxes from any payment or credit made under or with respect to the Notes, the Company will pay such additional amounts (the "Additional Amounts") as may be necessary so that the net payment or credit received by each owner of a beneficial interest in the Notes (including Additional Amounts) after such withholding or deduction will not be less than the amount the Holder or owner of a beneficial interest in the Notes would have received if such Taxes had not been withheld or deducted; provided that no Additional Amounts will be payable with respect to a payment or credit made to an owner of a beneficial interest in the Notes (i) which is subject to such Taxes by reason of its being connected with the Cayman Islands (or any successor jurisdiction) or any political subdivision thereof otherwise than by the mere holding, use or ownership or deemed holding, use or ownership of the Notes or the receipt of payments or credits or enforcing any rights thereunder, (ii) which is subject to such Taxes by reason of its failure to comply with any certification, identification, information, documentation or other reporting requirement if compliance is required by law, regulation, administrative practice or an applicable treaty as a precondition to exemption from, or a reduction in the rate of deduction or withholding of, such Taxes, (iii) which failed to duly and timely comply with a timely request by the Company to provide information, documents, certification or other evidence concerning such Holder's nationality, residence, entitlement to treaty benefits, identity or connection with the Cayman Islands (or such successor jurisdiction) or any political subdivision or authority thereof, if and to the extent that due and timely compliance with such request could have resulted in the reduction or elimination of any Taxes as to which Additional Amounts would otherwise have been payable to such Holder of Notes but for this clause (iii), (iv) which is a fiduciary, a partnership or not the beneficial owner of any payment on a Note, if and to the extent that any beneficiary or settlor of such fiduciary, any partner of such partnership or the beneficial owner of such payment (as the case may be) would not have been entitled to receive Additional Amounts with respect to such payment if
127
such beneficiary, settlor, partner or beneficial owner had been the Holder of such Note or (v) any combination of the foregoing clauses (i) through (iv) (in each case referred to herein as an "Excluded Holder"). The Company will also (1) make such withholding or deduction and (2) remit the full amount deducted or withheld to the relevant authority in accordance with and in the time required by applicable law. The Company will furnish the Holders of the Notes, within 30 days after the date the payment of any Taxes is due pursuant to applicable law, certified copies of tax receipts evidencing such payment by the Company, if reasonably available. In the event that the Company fails to remit any taxes in respect of which Additional Amounts are payable, the Company will indemnify and hold harmless each owner of a beneficial interest in the Notes (other than an Excluded Holder) and upon written request reimburse such owner of a beneficial interest in the Notes for the amount of (i) any Taxes levied on and paid by, such owner of a beneficial interest in the Notes as a result of payment made with respect to the Notes (including penalties, interest and expenses arising from or with respect to such Taxes) and (ii) any Taxes (including penalties, interest and expenses arising from or with respect to such Taxes) imposed with respect to payment of Additional Amounts or any reimbursement pursuant to this sentence.
At least 30 days prior to each date on which any payment under or with respect to the Notes is due and payable, if the Company will be obligated to pay Additional Amounts with respect to such payments, the Company will deliver to the Trustee an Officers' Certificate stating the fact that such Additional Amounts will be payable and the amounts so payable and setting forth such other information necessary to enable the Trustee to pay such Additional Amounts to Holders or owners of a beneficial interest in the Notes, as the case may be, on the payment date.
Events of Default and Remedies
The Indenture defines an "Event of Default" as:
128
The Indenture provides that if a Default occurs and is continuing, the Trustee must, within 90 days after receipt of notice of such Default, give to the Holders notice of such Default.
If an Event of Default occurs and is continuing (other than an Event of Default specified in clause (5) above relating to us or any of our Significant Subsidiaries), then in every such case, unless the principal of all of the Notes shall have already become due and payable, either the Trustee or the Holders of at least 25% in aggregate principal amount of the Notes then outstanding, by notice in writing to us (and to the Trustee if given by Holders) (an "Acceleration Notice"), may declare all principal, determined as set forth below, and accrued interest (and Liquidated Damages, if any) thereon to be due and payable immediately; provided, however, that if any Indebtedness is outstanding pursuant to the Credit Agreement, upon a declaration of such acceleration, such principal and interest shall be due and payable upon the earlier of (x) the fifth Business Day after sending us and the representative such written notice, unless such Event of Default is cured or waived prior to such date and (y) the date of acceleration of any Indebtedness outstanding under the Credit Agreement. In the event a declaration of acceleration resulting from an Event of Default described in clause (6) above with respect to any Indebtedness outstanding under the Credit Agreement has occurred and is continuing, such declaration of acceleration shall be automatically annulled if such default is cured or waived or the holders of the Indebtedness which is the subject of such default have rescinded their declaration of acceleration in respect of such Indebtedness within 30 days thereof and the Trustee has received written notice of such cure, waiver or rescission and no other Event of Default described in clause (6) above has occurred that has not been cured or waived within 30 days of the declaration of such acceleration in respect of such Indebtedness. If an Event of Default specified in clause (5), above, relating to the Company or any Significant Subsidiaries occurs, all principal and accrued interest (and Liquidated Damages, if any) thereon will be immediately due and payable on all Outstanding Notes without any declaration or other act on the part of the Trustee or the Holders. The Holders of a majority in aggregate principal amount of Notes generally are authorized to rescind such acceleration if all existing Events of Default, other than the non-payment of the principal of, premium, if any, and interest on the Notes which have become due solely by such acceleration have been cured or waived.
Prior to the declaration of acceleration of the maturity of the Notes, the Holders of a majority in aggregate principal amount of the Notes at the time outstanding may waive on behalf of all the Holders any Default, except a Default in the payment of principal of or interest on any Note not yet cured or a Default with respect to any covenant or provision which cannot be modified or amended without the consent of the Holder of each Outstanding Note affected. Subject to the provisions of the Indenture relating to the duties of the Trustee, the Trustee will be under no obligation to exercise any of its rights or powers under the Indenture at the request, order or direction of any of the Holders, unless such Holders have offered to the Trustee reasonable security or indemnity.
Subject to all provisions of the Indenture and applicable law, the Holders of a majority in aggregate principal amount of the Notes at the time outstanding will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred on the Trustee.
Legal Defeasance and Covenant Defeasance
The Indenture provides that we may, at our option, elect to discharge our obligations with respect to the Outstanding Notes ("Legal Defeasance"). If Legal Defeasance occurs, we shall be deemed to
129
have paid and discharged all amounts owed under the Notes, and the Indenture shall cease to be of further effect as to the Notes, except that:
In addition, we may, at our option and at any time, elect to cause the release of our obligations with respect to most of the covenants in the Indenture (except as described otherwise therein) ("Covenant Defeasance"). If Covenant Defeasance occurs, certain events (not including non-payment and bankruptcy, receivership, rehabilitation and insolvency events) relating to the Company, Capital or any Significant Subsidiary of the Company described under "Events of Default" will no longer constitute Events of Default with respect to the Notes. We may exercise Legal Defeasance regardless of whether we previously exercised Covenant Defeasance.
In order to exercise either Legal Defeasance or Covenant Defeasance (each, a "Defeasance"):
in either case to the effect that Holders of Notes will not recognize income, gain or loss for federal income tax purposes as a result of the Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if the Defeasance had not occurred;
130
The Defeasance will be effective on the earlier of (i) the 91st day after the deposit, and (ii) the day on which all the conditions above have been satisfied.
If the amount deposited with the Trustee to effect a Covenant Defeasance is insufficient to pay the principal of, premium, if any, and interest on the Notes when due, or if any court enters an order directing the repayment of the deposit to us or otherwise making the deposit unavailable to make payments under the Notes when due, then (so long as the insufficiency exists or the order remains in effect) our obligations under the Indenture and the Notes will be revived, and the Defeasance will be deemed not to have occurred.
Amendments and Supplements
The Indenture contains provisions permitting us and the Trustee to enter into a supplemental indenture for certain limited purposes without the consent of the Holders. With the consent of the Holders of not less than a majority in aggregate principal amount of the Notes at the time outstanding, we and the Trustee are permitted to amend or supplement the Indenture or any supplemental indenture or modify the rights of the Holders; provided, that no such modification may, without the consent of each Holder affected thereby:
Governing Law
The Indenture provides that it and the Notes will be governed by, and construed in accordance with, the laws of the State of New York including, without limitation, Sections 5-1401 and 5-1402 of the New York General Obligations Law and New York Civil Practice Laws and Rules 327(b).
131
No Personal Liability of Partners, Shareholders, Officers, Directors
The Indenture provides that no direct or indirect shareholder, employee, officer or director, as such, past, present or future of the Issuers or any successor entity shall have any liability in respect of our obligations under the Indenture or the Notes solely by reason of his or its status as such shareholder, employee, officer or director.
Certain Definitions
"Acquired Indebtedness" means Indebtedness (including Disqualified Capital Stock) of any Person existing at the time such Person becomes a Subsidiary of the Company, including by designation, or is merged or consolidated into or with the Company or a Subsidiary of the Company.
"Acquisition" means the purchase or other acquisition of any Person or all or substantially all the assets of any Person by any other Person, whether by purchase, merger, consolidation, or other transfer, and whether or not for consideration.
"Affiliate" means any Person directly or indirectly controlling or controlled by or under direct or indirect common control with the Company. For purposes of this definition, the term "control" means the power to direct the management and policies of a Person, directly or through one or more intermediaries, whether through the ownership of voting securities, by contract, or otherwise; provided, that with respect to ownership interest in the Company and its Subsidiaries, a Beneficial Owner of 10% or more of the total voting power normally entitled to vote in the election of directors, managers or trustees, as applicable, shall for such purposes be deemed to possess control.
"Applicable Premium" means, with respect to the Notes to be redeemed at any Early Redemption Date the excess of (A) the present value at such time of (i) the redemption price of such Notes at April 1, 2008 plus (ii) all interest required to be paid on such Notes from the date of redemption through April 1, 2008, computed using a discount rate equal to the Treasury Rate on such Early Redemption Date plus 0.75% per annum, over (B) the principal amount of such Notes.
"Applicable Tax Rate" means, in respect of any particular Tax Determination Year, a percentage equal to the highest marginal United States federal income tax rate applicable to an individual in respect of such Tax Determination Year as determined by the Tax Amounts CPA.
"Arm's-length Basis" means for any transaction between and among any member of the Holdings CFC Group and the Parent Group the pricing of which is determined on an arm's-length basis and in compliance with the "best method rule" and the "documentation requirements" under Sections 482 and 6662 of the Code and the Treasury regulations promulgated thereunder.
"Average Life" means, as of the date of determination, with respect to any security or instrument, the quotient obtained by dividing (1) the sum of the products (a) of the number of years from the date of determination to the date or dates of each successive scheduled principal (or redemption) payment of such security or instrument and (b) the amount of each such respective principal (or redemption) payment by (2) the sum of all such principal (or redemption) payments.
"Beneficial Owner" or "beneficial owner" for purposes of the definition of Change of Control and Affiliate has the meaning attributed to it in Rules 13d-3 and 13d-5 under the Exchange Act (as in effect on the Issue Date), whether or not applicable.
"Board of Directors" means, with respect to any Person, the board of directors (or if such Person is not a corporation, the equivalent board of managers or members or body performing similar functions for such Person) of such Person or any committee of the Board of Directors of such Person authorized, with respect to any particular matter, to exercise the power of the board of directors of such Person.
132
"Business Day" means each Monday, Tuesday, Wednesday, Thursday and Friday which is not a day on which banking institutions in New York, New York are authorized or obligated by law or executive order to close.
"Capital" means WH Capital Corporation, a Nevada corporation.
"Capital Contribution" means any contribution to the equity of the Company from the holders of the Company's Equity Interests for which no consideration other than the issuance of Qualified Capital Stock is given.
"Capitalized Lease Obligation" means, as to any Person, the obligations of such Person under a lease that are required to be classified and accounted for as capital lease obligations under GAAP and, for purposes of this definition, the amount of such obligations at any date shall be the capitalized amount of such obligations at such date, determined in accordance with GAAP.
"Capital Stock" means, with respect to any corporation, any and all shares, interests, rights to purchase (other than convertible or exchangeable Indebtedness that is not itself otherwise capital stock), warrants, options, participations or other equivalents of or interests (however designated) in stock issued by that corporation.
"Cash Equivalent" means:
and in the case of each of (1) and (2) maturing within one year after the date of acquisition.
"Code" means the Internal Revenue Code of 1986, as amended.
"Company" means WH Holdings (Cayman Islands) Ltd., an exempted company with limited liability organized under the laws of the Cayman Islands.
133
"Consolidated Coverage Ratio" of any Person on any date of determination (the "Transaction Date") means the ratio, on a pro forma basis, of (a) the aggregate amount of Consolidated EBITDA of such Person attributable to continuing operations and businesses (exclusive of amounts attributable to operations and businesses permanently discontinued or disposed of) for the Reference Period to (b) the aggregate Consolidated Fixed Charges of such Person (exclusive of amounts attributable to operations and businesses permanently discontinued or disposed of, but only to the extent that the obligations giving rise to such Consolidated Fixed Charges would no longer be obligations contributing to such Person's Consolidated Fixed Charges subsequent to the Transaction Date) during the Reference Period; provided, that for purposes of such calculation:
"Consolidated EBITDA" means, with respect to any Person, for any period, the Consolidated Net Income of such Person for such period adjusted to add thereto (to the extent deducted from net revenues in determining Consolidated Net Income), without duplication, the sum of
less the amount of all cash payments made by such Person or any of its Subsidiaries during such period to the extent such payments relate to non-cash charges that were added back in determining Consolidated EBITDA for such period or any prior period; provided, that consolidated income tax
134
expense and depreciation and amortization of a Subsidiary that is a less than Wholly-Owned Subsidiary shall only be added to the extent of the equity interest of the Company in such Subsidiary.
"Consolidated Fixed Charges" of any Person means, for any period, the aggregate amount (without duplication and determined in each case in accordance with GAAP) of:
For purposes of this definition, (x) interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined in good faith by the Company to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP and (y) interest expense attributable to any Indebtedness represented by the guaranty by such Person or a Subsidiary of such Person of an obligation of another Person shall be deemed to be the interest expense attributable to the Indebtedness guaranteed.
"Consolidated Net Income" means, with respect to any Person for any period, the net income (or loss) of such Person and its Consolidated Subsidiaries (determined on a consolidated basis in accordance with GAAP) for such period, reduced by the amount of any Tax Amounts Payments made during such period and adjusted to exclude (only to the extent included in computing such net income (or loss) and without duplication):
"Consolidated Subsidiary" means, for any Person, each Subsidiary of such Person (whether now existing or hereafter created or acquired) the financial statements of which are consolidated for financial statement reporting purposes with the financial statements of such Person in accordance with GAAP.
"Consolidation" means, with respect to the Company, the consolidation of the accounts of the Subsidiaries with those of the Company, all in accordance with GAAP; provided, that "consolidation" will not include consolidation of the accounts of any Unrestricted Subsidiary with the accounts of the Company. The term "consolidated" has a correlative meaning to the foregoing.
"Continuing Director" means during any period of 12 consecutive months after the Issue Date, individuals who at the beginning of any such 12-month period constituted the Board of Directors of the Company (together with any new directors whose election by such Board of Directors or whose nomination for election by the shareholders of the Company was approved by a vote of a majority of the directors then still in office who were either directors at the beginning of such period or whose
135
election or nomination for election was previously so approved, including new directors designated in or provided for in an agreement regarding the merger, consolidation or sale, transfer or other conveyance, of all or substantially all of the assets of the Company, if such agreement was approved by a vote of such majority of directors).
"Credit Agreement" means the credit agreement dated June 27, 2002, by and among Herbalife International, Inc., the Company, certain Subsidiaries of the Company, certain financial institutions and UBS AG Stamford Branch, as agent, providing for a term loan facility and a revolving credit facility, including any related notes, guarantees, collateral documents, instruments and agreements executed in connection therewith, as such credit agreement and/or related documents may be amended, restated, supplemented, renewed, replaced or otherwise modified from time to time whether or not with the same agent, trustee, representative lenders or holders, and, subject to the proviso to the next succeeding sentence, irrespective of any changes in the terms and conditions thereof. Without limiting the generality of the foregoing, the term "Credit Agreement" shall include agreements in respect of Interest Swap and Hedging Obligations with lenders (or Affiliates thereof) party to the Credit Agreement and shall also include any amendment, amendment and restatement, renewal, extension, restructuring, supplement or modification to any Credit Agreement and all refundings, refinancings and replacements of any Credit Agreement, including any credit agreement:
"Default" means any event that is or with the passage of time or the giving of notice or both would be an Event of Default.
"Disqualified Capital Stock" means with respect to the Company, (a) Equity Interests of the Company that, by its terms or by the terms of any security into which it is convertible, exercisable or exchangeable, is, or upon the happening of an event or the passage of time or both would be, required to be redeemed or repurchased including at the option of the holder thereof by the Company or any of its Subsidiaries, in whole or in part, on or prior to 91 days following the Stated Maturity of the Notes and (b) any Equity Interests of the Company or of any Subsidiary of the Company other than any common equity with no preferences, privileges, and no redemption or repayment provisions. Notwithstanding the foregoing, any Equity Interests that would constitute Disqualified Capital Stock solely because the holders thereof have the right to require the Company to repurchase such Equity Interests upon the occurrence of a change of control or an asset sale shall not constitute Disqualified Capital Stock if the terms of such Equity Interests provide that the Company may not repurchase or redeem any such Equity Interests pursuant to such provisions prior to the Company's purchase of the Notes as are required to be purchased pursuant to the provisions of the Indenture as described under "Repurchase at the Option of Holders."
"Equity Interests" means Capital Stock or partnership, participation or membership interests and all warrants, options or other rights to acquire Capital Stock or partnership, participation or membership interests (but excluding any debt security that is convertible into, or exchangeable for, Capital Stock or partnership, participation or membership interests).
136
"Exchange Act" means the Securities Exchange Act of 1934, as amended.
"Exempted Affiliate Transaction" means (a) customary employee compensation arrangements approved by a majority of independent (as to such transactions) members of the Board of Directors of the Company, (b) Restricted Payments permitted under the terms of the covenant discussed above under "—Limitation on Restricted Payments" above, (c) transactions solely between or among the Company and any Subsidiaries of the Company or solely among Subsidiaries of the Company, (d) so long as no Default or Event of Default has occurred and is continuing at the time of such payment, payment of Monitoring Fees pursuant to the Monitoring Services Agreements, (e) payment of any Tax Amounts Payments that are not prohibited by the covenant described above under the caption "—Limitation on Restricted Payments," (f) the Monitoring Services Agreements, (g) Capital Contributions to the Company or any sale of Capital Stock (other than Disqualified Capital Stock) of the Company to an Affiliate and (h) payment of reasonable directors' fees and customary indemnification and insurance agreements in favor of directors.
"Existing Indebtedness" means Indebtedness (including unfunded commitments therefor) of the Company and its Subsidiaries in existence on the Issue Date, reduced to the extent such amounts are repaid, refinanced or retired.
"Final Determination" means a final "determination" as defined under section 1313 of the Code or a similar determination under state, local, or foreign law.
"Final Determination Amount" means, in respect of any particular Tax Determination Year, any additional taxes, interest, and penalties resulting from a Final Determination and arising from or attributable to amounts paid or accrued pursuant to the Intercompany Subpart F Income.
"Foreign Subsidiary" means any Subsidiary of the Company which (i) is not organized under the laws of the United States, any state thereof or the District of Columbia and (ii) conducts substantially all of its business operations outside the United States of America.
"GAAP" means United States generally accepted accounting principles set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as approved by a significant segment of the accounting profession in the United States as in effect at the time.
"Guarantor" means (a) any Subsidiary Guarantor and (b) any other Subsidiary of the Company that has guaranteed the Company's obligations under the Indenture in accordance with the terms of the Indenture.
"Herbalife" means Herbalife International, Inc., a Nevada corporation, and its successors.
"Herbalife Notes" means the 113/4% Senior Subordinated Notes due 2011 of Herbalife.
"Holdings CFC Group" means the Company and any direct or indirect Subsidiary of the Company other than Herbalife and any direct or indirect Subsidiary of Herbalife.
"Holdings Group" means the Company and its Subsidiaries.
"Indebtedness" of any Person means, without duplication,
137
of the purchase price of any property or services, except those incurred in the ordinary course of its business that would constitute ordinarily a trade payable to trade creditors;
For purposes hereof, the "maximum fixed repurchase price" of any Disqualified Capital Stock which does not have a fixed repurchase price shall be calculated in accordance with the terms of such Disqualified Capital Stock as if such Disqualified Capital Stock were purchased on any date on which Indebtedness shall be required to be determined pursuant to the Indenture, and if such price is based upon, or measured by, the Fair Market Value of such Disqualified Capital Stock, such Fair Market Value to be determined in good faith by the board of directors of the issuer (or managing general partner of the issuer) of such Disqualified Capital Stock.
The amount of any Indebtedness outstanding as of any date shall be (1) the accreted value thereof, in the case of any Indebtedness issued with original issue discount, but the accretion of original issue discount in accordance with the original terms of Indebtedness issued with an original issue discount will not be deemed to be an incurrence and (2) the principal amount thereof, in the case of any other Indebtedness.
"Initial Public Offering" means an underwritten public offering of common stock of the Company in which gross proceeds to the Company are at least $50.0 million.
"Intercompany Subpart F Income" means, in respect of any Tax Determination Year, (i) the subpart F income of any member of the Holdings CFC Group for such year as determined under section 951(a)(1)(A) of the Code and (ii) the amount of earnings of any member of the Holdings CFC Group for such year as determined under section 951(a)(1)(B) of the Code in respect of any section 956 amount on income derived by the Holdings CFC Group in the ordinary course of its commercial activities conducted on an Arm's-length Basis with the Parent Group.
"Interest Swap and Hedging Obligation" means any obligation of any Person pursuant to any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, interest rate exchange agreement, currency exchange agreement or any other agreement or arrangement designed to protect against fluctuations in interest rates or currency values, including, without limitation, any arrangement whereby, directly or indirectly, such Person is entitled to receive from time to time periodic payments calculated by applying either a fixed or floating rate of interest on a stated notional amount in exchange for periodic payments made by such Person calculated by applying a fixed or floating rate of interest on the same notional amount.
138
"Investment" by any Person in any other Person means (without duplication):
The Company, without duplication, shall be deemed to make an Investment in an amount equal to the fair market value of the net assets of any subsidiary of the Company (or, if neither the Company nor any of its Subsidiaries has theretofore made an Investment in such subsidiary, in an amount equal to the Investments being made), at the time that such subsidiary is designated an Unrestricted Subsidiary, and any property transferred to an Unrestricted Subsidiary from the Company or a Subsidiary of the Company shall be deemed an Investment valued at its fair market value at the time of such transfer.
"Issue Date" means the date of first issuance of the Notes under the Indenture.
"Junior Security" means any Qualified Capital Stock and any Indebtedness of the Company or a Guarantor, as applicable, that is contractually subordinated in right of payment to Indebtedness outstanding under the Credit Agreement at least to the same extent as the Notes, and has no scheduled installment of principal due, by redemption, sinking fund payment or otherwise, on or prior to the Stated Maturity of the Notes; provided, that "Junior Security" shall mean any Qualified Capital Stock and any Indebtedness of the Company or the Guarantor, as applicable, that:
"Lien" means any mortgage, charge, pledge, lien (statutory or otherwise), privilege, security interest, hypothecation or other encumbrance upon or with respect to any property of any kind, real or personal, movable or immovable, now owned or hereafter acquired.
139
"Limited Debtor Subsidiary" means any Subsidiary of the Company that has no material Indebtedness other than the Notes as a guaranty of the Notes, and /or a guarantee with respect to the Herbalife Notes, the Credit Agreement and/or the Notes.
"Liquidated Damages" means all liquidated damages then owing pursuant to the Registration Rights Agreement.
"Luxembourg Holdings" means WH Luxembourg Holdings SàRL, a Luxembourg company, and its successors and/or assigns.
"Luxembourg Intermediate Holdings" means WH Luxembourg Intermediate Holdings SàRL, a Luxembourg company, and its successors and/or assigns.
"Monitoring Fees" means payments to Whitney or GGC Administration, LLC pursuant to the Monitoring Services Agreements.
"Monitoring Services Agreements" means those certain separate monitoring fee agreements dated as of July 31, 2002, among (i) the Company, Herbalife and Whitney and (ii) the Company, Herbalife and GGC Administration, LLC, without giving effect to any amendment thereto or waiver thereof.
"Net Cash Proceeds" means the aggregate amount of cash or Cash Equivalents received by the Company in the case of a sale, or Capital Contribution in respect, of Qualified Capital Stock and by the Company and the Subsidiaries of the Company in respect of an Asset Sale plus, in the case of an issuance of Qualified Capital Stock upon any exercise, exchange or redemption of securities (including options, warrants, rights and convertible or exchangeable debt) of the Company that were issued for cash on or after the Issue Date, the amount of cash originally received by the Company upon the issuance of such securities (including options, warrants, rights and convertible or exchangeable debt) less, in each case, the sum of all payments, fees, commissions and (in the case of Asset Sales, reasonable and customary), expenses (including, without limitation, the fees and expenses of legal counsel and investment banking fees and expenses) incurred in connection with such Asset Sale or sale of Qualified Capital Stock, and, in the case of an Asset Sale only, less the amount (estimated reasonably and in good faith by the Company) of income, franchise, sales and other applicable taxes required to be paid by the Company or any Subsidiary of the Company in connection with such Asset Sale in the taxable year that such sale is consummated or in the immediately succeeding taxable year, the computation of which shall take into account the reduction in tax liability resulting from any available operating losses and net operating loss carryovers, tax credits and tax credit carryforwards, and similar tax attributes, less amounts required to be applied to the repayment of Indebtedness, other than Indebtedness outstanding under the Credit Agreement, secured by a Lien on the assets or assets that were the subject of the Asset Sale.
"Notice of Deficiency" means a notice of deficiency as described under section 6212 of the Code or a similar notice under state, local, or foreign law.
"Obligation" means any principal, premium or interest payment, or monetary penalty, or damages, due by the Issuers or any Guarantor under the terms of the Notes or the Indenture, including any Liquidated Damages due pursuant to the terms of the Registration Rights Agreement.
"Officers' Certificate" means the officers' certificate to be delivered upon the occurrence of certain events as set forth in the Indenture.
"Parent" means WH Intermediate Holdings Ltd., a Cayman Islands corporation.
"Parent Group" means Parent and its subsidiaries.
"Parent Group Tax Savings Amount" means, in respect of any Tax Determination Year, the excess of (x) the tax liability incurred by the Parent Group for such Tax Determination Year as determined as if Herbalife had earned the Intercompany Subpart F Income of the Holdings CFC Group arising in the
140
ordinary course of the commercial activities, conducted on an Arm's-length Basis, between the Parent Group and the Holdings CFC Group over (y) the actual tax liability incurred by the Parent Group for such Tax Determination Year (as determined on a basis consistent with any Final Determination in respect of any previous Tax Determination Year), which liability shall take into account any taxes that have been, or will be, incurred by the Parent Group in connection with the making of a Tax Amounts Payment in respect of such Tax Determination Year. If, in respect of any Tax Determination Year, the Parent or any Subsidiary of the Parent Group has received a Notice of Deficiency, in respect of which there has been no Final Determination, related to any item arising from or attributable to amounts paid or accrued pursuant to the Intercompany Subpart F Income, the Parent Group Tax Savings Amount shall be determined on a basis consistent with such Notice of Deficiency except to the extent that, based on the advice of the Tax Amounts CPA, the Parent reasonably determines that, more likely than not, the Parent or such Subsidiary will prevail on the merits in connection with contesting such Notice of Deficiency.
"Permitted Indebtedness" means that:
141
"Permitted Investment" means:
"Permitted Jurisdiction" means the United States, and any state thereof and the District of Columbia, any member country of the European Union, Bermuda, the British Virgin Islands, Gibraltar, Hong Kong, Switzerland and Singapore.
"Permitted Lien" means:
142
and the Lien is not extended to any additional assets or property that would not have been security for the Indebtedness refinanced; and
"Person" or "person" means any corporation, individual, limited liability company, joint stock company, joint venture, partnership, limited liability company, unincorporated association, governmental regulatory entity, country, state or political subdivision thereof, trust, municipality or other entity.
"Preferred Stock" means any Equity Interest of any class or classes of a Person (however designated) which is preferred as to payments of dividends, or as to distributions upon any liquidation or dissolution, over Equity Interests of any other class of such Person.
"Pro Forma" or "pro forma" shall have the meaning set forth in Regulation S-X of the Securities Act of 1933, as amended, unless otherwise specifically stated herein.
"Principals" means each of (1) Whitney V, L.P. and (2) CCG Investments (BVI), L.P.
"Purchase Money Indebtedness" of any Person means any Indebtedness of such Person to any seller or other Person incurred solely to finance the acquisition (including in the case of a Capitalized Lease Obligation, the lease), construction, installation or improvement of any after acquired real or personal tangible property which, in the reasonable good faith judgment of the Board of Directors of the Company, is directly related to a Related Business of the Company and which is incurred substantially concurrent with such acquisition, construction, installation or improvement and is secured only by the assets so financed.
"Qualified Capital Stock" means any Capital Stock of the Company that is not Disqualified Capital Stock.
"Qualified Equity Offering" means, (i) an underwritten public offering pursuant to a registration statement filed with the Commission in accordance with the Securities Act of 1933, as amended, of Qualified Capital Stock of the Company, or (ii) an unregistered offering of Qualified Capital Stock of the Company for cash resulting in net proceeds to the Company in excess of $50.0 million.
"Qualified Exchange" means:
"Recourse Indebtedness" means Indebtedness (a) as to which either the Company or any Subsidiary of the Company (1) provides credit support of any kind (including any undertaking, agreement or instrument that would constitute Indebtedness), (2) is directly or indirectly liable (as a guarantor or otherwise), or (3) constitutes the lender, and (b) a default with respect to which (including any rights that the holders thereof may have to take enforcement action against an Unrestricted Subsidiary) would permit (upon notice, lapse of time or both) any holder of any other Indebtedness of the Company or any Subsidiary of the Company to declare a default on such other Indebtedness or cause the payment thereof to be accelerated or payable prior to its stated maturity.
143
"Reference Period" with regard to any Person means the four full fiscal quarters ended immediately preceding any date upon which any determination is to be made pursuant to the terms of the Notes or the Indenture.
"Refinancing Indebtedness" means Indebtedness (including Disqualified Capital Stock) (a) issued in exchange for, or the proceeds from the issuance and sale of which are used substantially concurrently to repay, redeem, defease, refund, refinance, discharge or otherwise retire for value, in whole or in part, or (b) constituting an amendment, modification or supplement to, or a deferral or renewal of ((a) and (b) above are, collectively, a "Refinancing"), any Indebtedness (including Disqualified Capital Stock) in a principal amount or, in the case of Disqualified Capital Stock, liquidation preference, not to exceed (after deduction of reasonable and customary fees and expenses incurred in connection with the Refinancing plus the amount of any premium paid in connection with such Refinancing) the lesser of (1) the principal amount or, in the case of Disqualified Capital Stock, liquidation preference, of the Indebtedness (including Disqualified Capital Stock) so Refinanced and (2) if such Indebtedness being Refinanced was issued with an original issue discount, the accreted value thereof (as determined in accordance with GAAP) at the time of such Refinancing; provided, that (A) such Refinancing Indebtedness shall only be used to refinance outstanding Indebtedness (including Disqualified Capital Stock) of such Person issuing such Refinancing Indebtedness, (B) such Refinancing Indebtedness shall (x) not have an Average Life shorter than the Indebtedness (including Disqualified Capital Stock) to be so refinanced at the time of such Refinancing and (y) in all respects, be no less contractually subordinated or junior, if applicable, to the rights of Holders than was the Indebtedness (including Disqualified Capital Stock) to be refinanced, (C) such Refinancing Indebtedness shall have a final stated maturity or redemption date, as applicable, no earlier than the final stated maturity or redemption date, as applicable, of the Indebtedness (including Disqualified Capital Stock) to be so refinanced or, if sooner, 91 days after the Stated Maturity of the Notes, and (D) such Refinancing Indebtedness shall be secured (if secured) in a manner no more adverse to the Holders than the terms of the Liens (if any) securing such refinanced Indebtedness, including, without limitation, the amount of Indebtedness secured shall not be increased.
"Registration Rights Agreement" means the Registration Rights Agreement, dated as of the Issue Date, by and among the Company and the other parties named on the signature pages thereof, as such agreement may be amended, modified or supplemented from time to time.
"Related Business" means the business conducted (or proposed to be conducted) by the Company and its Subsidiaries as of the Issue Date and any and all businesses that in the good faith judgment of the Board of Directors of the Company are materially related, ancillary or complementary businesses.
"Related Business Asset" means assets that the Company determines will be used in a Related Business.
"Related Party" means, with respect to any of the Principals, any Person who controls, is controlled by or is under common control with such Principal; provided, that for purposes of this definition "control" means the beneficial ownership of more than 80% of the total voting power of a Person normally entitled to vote in the election of directors, managers or trustees, as applicable of a Person.
"Restricted Investment" means, in one or a series of related transactions, any Investment, other than other Permitted Investments.
"Restricted Payment" means, with respect to any Person:
144
provided, however, that the term "Restricted Payment" does not include (1) any dividend, distribution or other payment on or with respect to Equity Interests of an issuer to the extent payable solely in shares of Qualified Capital Stock of such issuer, or (2) any dividend, distribution or other payment to the Company, or to any Subsidiary, by the Company, any Subsidiary of the Company, or (3) any Investment in the Company or any Subsidiary of the Company; provided, that the consideration for such Investment will be received by the Company or any Subsidiary, or (4) the repurchase of Equity Interests deemed to occur upon the exercise of stock options if such Equity Interests represent a portion of the exercise price thereof.
"Senior Indebtedness" means Indebtedness (including any obligation in respect of the Credit Agreement, and interest, whether or not allowable, accruing on Indebtedness incurred pursuant to the Credit Agreement after the filing of a petition initiating any proceeding under any bankruptcy, insolvency or similar law) of WH Intermediate or any of its Subsidiaries, whether outstanding on the Issue Date or thereafter incurred, arising under the Credit Agreement or any other Indebtedness (unless, in the case of any particular Indebtedness, the instrument creating or evidencing such Indebtedness expressly provides that such Indebtedness shall be contractually subordinated in right of payment to any other Indebtedness of the obligor thereunder); provided that in no event shall Senior Indebtedness include (a) Indebtedness to any Subsidiary of the Company or any Affiliate of the Company, (b) Indebtedness incurred in violation of the terms of the Indenture; provided that Indebtedness under the Credit Agreement will not cease to be Senior Indebtedness as a result of this clause (b) if the lenders thereunder obtained a certificate from an executive officer of the Company on the date such Indebtedness was incurred certifying that the incurrence of such Indebtedness was not prohibited by the Indenture, (c) Indebtedness to trade creditors, (d) Disqualified Capital Stock, (e) Capitalized Lease Obligations, unless designated in the instrument evidencing such Capitalized Lease Obligations as "Senior Indebtedness", (f) any amounts owed for goods, materials or services purchased in the ordinary course of business or for compensation to employees of the Company or any of its Subsidiaries and (g) any liability for taxes owed or owing.
"Significant Subsidiary" shall have the meaning provided under Regulation S-X of the Securities Act, as in effect on the Issue Date.
"Stated Maturity," when used with respect to any Note, means April 1, 2011.
"Subordinated Indebtedness" means Indebtedness of the Issuers that is subordinated in right of payment by its terms or the terms of any document or instrument or instrument relating thereto ("contractually") to the Notes.
"Subsidiary" with respect to any Person, means (1) a corporation a majority of whose Equity Interests with voting power, under ordinary circumstances, to elect directors is at the time, directly or indirectly, owned by such Person, by such Person and one or more Subsidiaries of such Person or by one or more Subsidiaries of such Person, and (2) any other Person (other than a corporation) in which such Person, one or more Subsidiaries of such Person, or such Person and one or more Subsidiaries of
145
such Person, directly or indirectly, at the date of determination thereof has a majority ownership interest, or (3) a partnership in which such Person or a Subsidiary of such Person is, at the time, a general partner and in which such Person, directly or indirectly, at the date of determination thereof has a majority ownership interest. Notwithstanding the foregoing, an Unrestricted Subsidiary shall not be a Subsidiary of the Company or of any Subsidiary of the Company. Unless the context requires otherwise, Subsidiary means each direct and indirect Subsidiary of the Company.
"Subsidiary Guarantor" means a Subsidiary of the Company that has jointly and severally irrevocably and unconditionally guaranteed the Notes, on a non-subordinated basis, except that such guarantee can be subordinated to such Subsidiary's guarantee of the Credit Agreement in a manner consistent with the subordination of the Company's obligations in respect of the Notes to the Company's Senior Indebtedness.
"Tax Amounts CPA" means PricewaterhouseCoopers L.L.P. or any other certified public accounting firm of national reputation. The Tax Amounts CPA shall reasonably determine for each Tax Determination Year, the Applicable Tax Rate, the Final Determination Amount, Intercompany Subpart F Income, Tax Amounts Payment and Parent Group Tax Savings Amount.
"Tax Amounts Payment" means, in respect of any Tax Determination Year, an amount payable to Tax Amounts Recipients equal to the lesser of (hereinafter referred to as the "Initial Limitation") (A) the product of (x) the Applicable Tax Rate and (y) the Intercompany Subpart F Income that is (or would be) includible in the gross income of the Tax Amounts Recipients (assuming, for this purpose, that each such Tax Amount Recipient is a "United States shareholder" as defined in section 951(b) of the Code) for such year under section 951(a) of the Code, (B) the Parent Group Tax Savings Amount for such year, (C) the product of (x) 6% and (y) the sum of (i) Consolidated Net Income of the Parent Group for such year, (ii) Consolidated income tax expense for the Parent Group for such year, and (iii) Tax Amount Payments made to Tax Amounts Recipients during such year, or (D) $10 million. The Initial Limitation shall be reduced (but not below zero) by any Final Determination Amount in respect of a previous Tax Determination Year. A Final Determination Amount shall be applied to reduce an Initial Limitation for the Tax Determination Year during which the Final Determination in respect of such Final Determination Amount occurs. A Final Determination Amount shall be deemed to be reduced to the extent that such Final Determination Amount has been applied to reduce an Initial Limitation. Thereafter, the remaining Final Determination Amount, if any, shall be applied to reduce the Initial Limitation for each successive Tax Determination Year in like fashion until such Final Determination Amount has been reduced to zero.
"Tax Amounts Recipient" means, in respect of any Tax Determination Year, persons who hold capital stock of the Company on December 31 of such year or, if earlier, on the last day of such year that the Company continues to be a "controlled foreign corporation" as defined under section 957 of the Code.
"Tax Determination Year" means the calendar year in respect of which a Tax Amounts Recipient is (or would be) required to include in gross income under section 951(a) of the Code his pro rata share of Intercompany Subpart F Income (assuming for this purpose, that such Tax Amounts Recipient is a "United States shareholder" as defined in Section 951(b) of the Code).
"Treasury Rate" means the yield to maturity at the time of computation of U.S. Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Release H 15 (519) which has become publicly available at least two Business Days prior to the Early Redemption Date (or, if such Statistical Release is no longer published, any publicly available source or similar market data) closest to the period from the Early Redemption Date to April 1, 2008; provided, however, that if the period from the Early Redemption Date to April 1, 2008 is not equal to the constant maturity of a U.S. Treasury security for which a weekly average is given, the Treasury Rate shall be obtained by linear interpolation (calculated to the nearest one-twelfth of one year) from the
146
weekly average yields of U.S. Treasury securities for which such yields are given, except that if the period from the Early Redemption Date to April 1, 2008 is less than one year, the weekly average yield on actually traded U.S. Treasury securities adjusted to a constant maturity of one year shall be used.
"UCC" means the Uniform Commercial Code as in effect from time to time in the State of New York.
"Unrestricted Subsidiary" means any subsidiary of the Company that does not directly, indirectly or beneficially own any Capital Stock of, and Subordinated Indebtedness of, or own or hold any Lien on any property of, the Company or any other Subsidiary of the Company and that, at the time of determination, shall be an Unrestricted Subsidiary (as designated by the Board of Directors of the Company); provided, that such Subsidiary at the time of such designation (a) has no Recourse Indebtedness; (b) is not party to any agreement, contract, arrangement or understanding with the Company or any Subsidiary of the Company unless the terms of any such agreement, contract, arrangement or understanding are no less favorable to the Company or such Subsidiary than those that might be obtained at the time from Persons who are not Affiliates of the Company; (c) is a Person with respect to which neither the Company nor any of Subsidiary of the Company has any direct or indirect obligation (x) to subscribe for additional Equity Interests or (y) to maintain or preserve such Person's financial condition or to cause such Person to achieve any specified levels of operating results; and (d) has not guaranteed or otherwise directly or indirectly provided credit support for any Indebtedness of the Company or any Subsidiary of the Company. The Board of Directors of the Company may designate any Unrestricted Subsidiary to be a Subsidiary, provided, that (1) no Default or Event of Default is existing or will occur as a consequence thereof and (2) immediately after giving effect to such designation, on a pro forma basis, we could incur at least $1.00 of Indebtedness pursuant to the Debt Incurrence Ratio of the covenant "Limitation on Incurrence of Additional Indebtedness and Disqualified Capital Stock." Each such designation shall be evidenced by filing with the Trustee a certified copy of the resolution giving effect to such designation and an Officers' Certificate certifying that such designation complied with the foregoing conditions.
"U.S. Government Obligations" means direct non-callable obligations of, or non-callable obligations guaranteed by, the United States of America for the payment of which obligation or guarantee the full faith and credit of the United States of America is pledged.
"Voting Equity Interests" means Equity Interests which at the time are entitled to vote in the election of, as applicable, directors, members or partners generally.
"WH Intermediate" means WH Intermediate Holdings Ltd., a Cayman Islands corporation, and its successors and/or assigns.
"Wholly-Owned Subsidiary" means a Subsidiary all the Equity Interests of which (other than directors' qualifying shares) are owned by the Company or one or more Wholly-Owned Subsidiaries of the Company.
Registration Rights; Liquidated Damages
The Issuers and the Initial Purchaser entered into the Registration Rights Agreement. In the Registration Rights Agreement, the Issuers agreed to file the exchange offer registration statement with the Commission within 105 days after the Issue Date and to use reasonable efforts to have it declared effective within 165 days after the Issue Date. The Issuers also agreed to use their reasonable efforts to cause the exchange offer registration statement to be effective continuously, to keep the exchange offer open for a period of not less than 30 days and cause the exchange offer to be consummated within 195 days after the Issue Date. Pursuant to the exchange offer, certain holders of the Outstanding Notes which constitute registrable Notes (as defined below) may exchange their registrable Notes for registered Notes. To participate in the exchange offer, each holder must represent among other things
147
that if it is an affiliate, it will comply with the registration and prospectus delivery requirements of the Securities Act to the extent applicable, it is not engaged in, and does not intend to engage in, and has no arrangement or understanding with any person to participate in, a distribution of the Notes that are issued in the exchange offer, and that it is acquiring the Notes in the exchange offer in its ordinary course of business.
If (i) the exchange offer is not permitted by changes in law or applicable interpretations of the staff of the Commission policy or (ii) for any reason the exchange offer is not consummated within 195 days after the Issue Date, (iii) a holder, other than the initial purchaser, is prohibited by law or the applicable interpretations of the staff of the Commission from participating in the exchange offer or does not receive exchange Notes on the date of the exchange that may be sold without restriction under state and federal securities laws or (iv) the initial purchaser so requests with respect to the Notes that have, or that are reasonably likely to be determined to have, the status of unsold allotments in an initial distribution, the Issuers will file with the Commission a shelf registration statement to register the registrable Notes for resale by holders in the manner designated by them.
For the purposes of the registration rights agreement, "registrable Notes" means each Outstanding Note until (i) a registration statement covering such note has been declared effective by the Commission and such note has been disposed of in accordance with such effective registration statement, (ii) such note has been exchanged pursuant to the exchange offer and may be resold without restriction under state and federal securities laws, (iii) such note ceases to be outstanding for purposes of the indenture or (iv) such note has been sold in compliance with Rule 144 or is salable pursuant to Rule 144(k) of the Securities Act.
The Registration Rights Agreement provides that (i) if the Issuers fail to file an exchange offer registration statement with the Commission on or prior to the 105th day after the Issue Date, (ii) if the exchange offer registration statement is not declared effective by the Commission on or prior to the 165th day after the Issue Date, (iii) if the exchange offer is not consummated on or before the 195th day after the Issue Date, (iv) if obligated to file a shelf registration statement and the shelf registration statement is not declared effective on or prior to the later of, the 195th calendar day after the Issue Date or the 90th day after the obligation to file a shelf registration statement arises, or (v) if the shelf registration statement is declared effective but thereafter ceases to be effective or useable in connection with resales of the registrable Notes, for such time of non-effectiveness or non-usability (each, a "registration default"), the Issuers agree to pay to each holder of registrable Notes affected thereby liquidated damages ("liquidated damages") in an amount equal to 0.25% per annum for the first 90-day period immediately following the occurrence of a registration default. The amount of the liquidated damages shall increase by an additional 0.25% per annum with respect to each subsequent 90-day period until all registration defaults have been cured, up to a maximum amount of liquidated damages of 1.00% per annum per week per $1,000 in principal amount of registrable Notes. The Issuers are not required to pay liquidated damages for more than one registration default at any given time. Following the cure of all registration defaults or when all the Notes and the exchange Notes otherwise become freely transferable by holders other than affiliates of the Issuers without further registration under the Securities Act, the accrual of liquidated damages will cease.
All accrued liquidated damages shall be paid semi-annually on the applicable Interest Payment Date by the Issuers to the Holders entitled thereto.
148
FORM AND TRANSFER OF THE NOTES
The Outstanding Notes sold to qualified institutional buyers are in the form of one or more registered global notes without interest coupons (collectively, the "Rule 144A Global Notes"). Upon issuance, the Rule 144A Global Notes were deposited with the Trustee, as custodian for The Depository Trust Company ("DTC"), in New York, New York, and registered in the name of DTC or its nominee for credit to the accounts of DTC's Direct and Indirect Participants (as defined below). The Outstanding Notes offered and sold in offshore transactions in reliance on Regulation S were in the form of one or more registered, temporary global book-entry notes without interest coupons (the "Reg S Global Notes"). The Reg S Global Notes were deposited with the Trustee, as custodian for DTC, in New York, New York and registered in the name of a nominee of DTC for credit to the accounts of Direct Participants and Indirect Participants (including the Euroclear System ("Euroclear") and Clearstream Banking, S.A. ("Clearstream"). During the 40-day period commencing on the day after the later of the offering and the Issue Date of the Outstanding Notes (the "40-Day Restricted Period"), Direct Participants and Indirect Participants that held a beneficial interest in the Reg S Global Notes were not able to transfer such interest to a person taking delivery thereof in the form of an interest in the Rule 144A Global Notes. After the 40 Day Restricted Period, (i) beneficial interests in the Reg S Global Notes may be transferred to a person that takes delivery in the form of an interest in the Rule 144A Global Notes and (ii) beneficial interests in the Rule 144A Global Notes may be transferred to a person that takes delivery in the form of an interest in the Reg S Global Notes, provided, in each case, that the certification requirements described below are complied with. See "—Transfers of Interests in One Global Note for Interests in Another Global Note."
New Notes
New Notes issued (i) in exchange for Outstanding Notes originally offered and sold to qualified institutional buyers in reliance on Rule 144A under the Securities Act or (ii) in reliance on Regulation S under the Securities Act will be represented by a single, permanent Global Note in definitive, fully registered book entry form (the "Exchange Global Note" and together with the Rule 144A Global Note and Reg S Global Note, the "Global Notes"), which will be registered in the name of DTC or its nominee on behalf of persons who receive New Notes represented thereby for credit to the respective accounts of such persons (or to such other accounts as they may direct) at DTC.
New Notes issued in exchange for Outstanding Notes will be issued, upon request, in fully registered form, but otherwise such holders will only be entitled to registration of their respective New Notes in book-entry form under the Exchange Global Note. See "Transfers of Interests in Global Notes for Certificated Notes" below.
The Global Notes
Transfer of beneficial interests in any Global Notes will be subject to the applicable rules and procedures of DTC and its Direct or Indirect Participants (including, if applicable, those of Euroclear and Clearstream), which may change from time to time.
The Global Notes may be transferred, in whole and not in part, only to another nominee of DTC or to a successor of DTC or its nominee in certain limited circumstances. Beneficial interests in the Global Notes may be exchanged for Notes in certificated form in certain limited circumstances. See "—Transfer of Interests in Global Notes for Certificated Notes."
Initially, the Trustee will act as Paying Agent and Registrar. The Notes may be presented for registration of transfer and exchange at the offices of the Registrar.
149
DTC has advised us that DTC is a limited-purpose trust company organized under the laws of the State of New York, a member of the Federal Reserve System, a "clearing corporation" within the meaning of the Uniform Commercial Code and a "clearing agency" registered pursuant to the provisions of Section 17A of the Exchange Act. DTC was created to hold securities for its participating organizations (collectively, the "Direct Participants") and to facilitate the clearance and settlement of transactions in those securities between Direct Participants through electronic book-entry changes in accounts of Participants. The Direct Participants include securities brokers and dealers (including the initial purchasers), banks, trust companies, clearing corporations and certain other organizations. Access to DTC's system is also available to other entities that clear through or maintain a direct or indirect, custodial relationship with a Direct Participant (collectively, the "Indirect Participants"), including Euroclear and Clearstream. DTC may hold securities beneficially owned by other persons only through the Direct Participants or Indirect Participants and such other person's ownership interest and transfer of ownership interest will be recorded only on the records of the Direct Participant and/or Indirect Participant and not on the records maintained by DTC.
DTC has advised us that, pursuant to DTC's procedures, (i) upon deposit of the Global Notes, DTC will credit the accounts of the Direct Participants with portions of the principal amount of the Global Notes that have been allocated to them, and (ii) DTC will maintain records of the ownership interests of such Direct Participants in the Global Notes and the transfer of ownership interests by and between Direct Participants. DTC will not maintain records of the ownership interests of, or the transfer of ownership interests by and between, Indirect Participants or other owners of beneficial interests in the Global Notes. Direct Participants and Indirect Participants must maintain their own records of the ownership interests of, and the transfer of ownership interests by and between, Indirect Participants and other owners of beneficial interests in the Global Notes.
Investors in the Rule 144A Global Notes may hold their interests therein directly through DTC if they are Direct Participants in DTC or indirectly through organizations that are Direct Participants in DTC. Investors in the Reg S Global Notes may hold their interests therein directly through DTC if they are Direct Participants in DTC, indirectly through organizations that have accounts with Direct Participants, including Euroclear or Clearstream, or indirectly through organizations that are participants in Euroclear or Clearstream. Euroclear Bank S.A./N.V. is the operator and depository of Euroclear and Citibank, N.A. is the operator and depository of Clearstream (each a "Nominee" of Euroclear and Clearstream, respectively). Therefore, they will each be recorded on DTC's records as the holders of all ownership interests held by them on behalf of Euroclear and Clearstream, respectively. Euroclear and Clearstream must maintain on their own records the ownership interests of, and transfers of ownership interests by and between, their own customers' securities accounts. DTC will not maintain such records. All ownership interests in any Global Notes, including those of customers' securities accounts held through Euroclear or Clearstream, may be subject to the procedures and requirements of DTC.
The laws of some states in the United States require that certain persons take physical delivery in definitive, certificated form, of securities that they own. This may limit or curtail the ability to transfer beneficial interests in a Global Note to such persons. Because DTC can act only on behalf of Direct Participants, which in turn act on behalf of Indirect Participants and others, the ability of a person having a beneficial interest in a Global Note to pledge such interest to persons or entities that are not Direct Participants in DTC, or to otherwise take actions in respect of such interests, may be affected by the lack of physical certificates evidencing such interests. For certain other restrictions on the transferability of the Notes see "—Transfers of Interests in Global Notes for Certificated Notes."
Except as described in "—Transfers of Interests in Global Notes for Certificated Notes," owners of beneficial interests in the Global Notes will not have Outstanding Notes or New Notes registered in their names, will not receive physical delivery of Outstanding Notes or New Notes in certificated form
150
and will not be considered the registered owners or holders thereof under the Indenture for any purpose.
Under the terms of the Indenture, we and the Trustee will treat the persons in whose names the Outstanding Notes or New Notes are registered (including notes represented by Global Notes) as the owners thereof for the purpose of receiving payments and for any and all other purposes whatsoever. Payments in respect of the principal, premium, Liquidated Damages, if any, and interest on Global Notes registered in the name of DTC or its nominee will be payable by the Trustee to DTC or its nominee as the registered holder under the Indenture. Consequently, neither we, the Trustee nor any agent of ours or the Trustee has or will have any responsibility or liability for (i) any aspect of DTC's records or any Direct Participant's or Indirect Participant's records relating to or payments made on account of beneficial ownership interests in the Global Notes or for maintaining, supervising or reviewing any of DTC's records or any Direct Participant's or Indirect Participant's records relating to the beneficial ownership interests in any Global Note or (ii) any other matter relating to the actions and practices of DTC or any of its Direct Participants or Indirect Participants.
DTC has advised us that its current payment practice (for payments of principal, interest and the like) with respect to securities such as the Outstanding Notes and the New Notes is to credit the accounts of the relevant Direct Participants with such payment on the payment date in amounts proportionate to such Direct Participant's respective ownership interests in the Global Notes as shown on DTC's records. Payments by Direct Participants and Indirect Participants to the beneficial owners of the Outstanding Notes and the New Notes will be governed by standing instructions and customary practices between them and will not be the responsibility of DTC, the Trustee, or us. Neither we nor the Trustee will be liable for any delay by DTC or its Direct Participants or Indirect Participants in identifying the beneficial owners of the Outstanding Notes or the New Notes, and we and the Trustee may conclusively rely on and will be protected in relying on instructions from DTC or its nominee as the registered owner of such notes for all purposes.
The Global Notes will trade in DTC's Same-Day Funds Settlement System and, therefore, transfers between Direct Participants in DTC will be effected in accordance with DTC's procedures, and will be settled in immediately available funds. Transfers between Indirect Participants (other than Indirect Participants who hold an interest in the Notes through Euroclear or Clearstream) who hold an interest through a Direct Participant will be effected in accordance with the procedures of such Direct Participant but generally will settle in immediately available funds. Transfers between and among Indirect Participants who hold interests in the Outstanding Notes or the New Notes through Euroclear and Clearstream will be effected in the ordinary way in accordance with their respective rules and operating procedures.
Subject to compliance with the transfer restrictions applicable to the notes described herein, cross-market transfers between Direct Participants in DTC, on the one hand, and Indirect Participants who hold interests in the Outstanding Notes or the New Notes through Euroclear or Clearstream, on the other hand, will be effected by Euroclear's or Clearstream's respective Nominee through DTC in accordance with DTC's rules on behalf of Euroclear or Clearstream; however, delivery of instructions relating to cross-market transactions must be made directly to Euroclear or Clearstream, as the case may be, by the counterparty in accordance with the rules and procedures of Euroclear or Clearstream and within their established deadlines. Indirect Participants who hold interests in the Outstanding Notes or the New Notes through Euroclear and Clearstream may not deliver instructions directly to Euroclear's or Clearstream's Nominee. Euroclear or Clearstream will, if the transaction meets its settlement requirements, deliver instructions to its respective Nominee to deliver or receive interests on Euroclear's or Clearstream's behalf in the relevant Global Note in DTC, and make or receive payment in accordance with normal procedures for same-day fund settlement applicable to DTC.
151
Because of time zone differences, the securities accounts of an Indirect Participant who holds an interest in the Outstanding Notes or the New Notes through Euroclear or Clearstream purchasing an interest in a Global Note from a Direct Participant in DTC will be credited, and any such crediting will be reported to Euroclear or Clearstream during the European business day immediately following the settlement date of DTC in New York. Although recorded in DTC's accounting records as of DTC's settlement date in New York, Euroclear and Clearstream customers will not have access to the cash amount credited to their accounts as a result of a sale of an interest in a Reg S Global Note to a DTC Participant until the European business day for Euroclear or Clearstream immediately following DTC's settlement date.
DTC has advised us that it will take any action permitted to be taken by a holder of New Notes or Outstanding Notes only at the direction of one or more Direct Participants to whose account interests in the Global Notes are credited and only in respect of such portion of the aggregate principal amount of the notes as to which such Direct Participant or Direct Participants has or have given direction. However, if there is an Event of Default under the Notes, DTC reserves the right to exchange Global Notes (without the direction of one or more of its Direct Participants) for legended Notes in certificated form, and to distribute such certificated forms of Notes to its Direct Participants. See "—Transfers of Interests in Global Notes for Certificated Notes."
Although DTC, Euroclear and Clearstream have agreed to the foregoing procedures to facilitate transfers of interests in the Reg S Global Notes and in the Rule 144A Global Notes among Direct Participants, including Euroclear and Clearstream, they are under no obligation to perform or to continue to perform such procedures, and such procedures may be discontinued at any time. None of us, the initial purchasers or the Trustee shall have any responsibility for the performance by DTC, Euroclear or Clearstream or their respective Direct and Indirect Participants of their respective obligations under the rules and procedures governing any of their operations.
The information in this section concerning DTC, Euroclear and Clearstream and their book-entry systems has been obtained from sources that we believe to be reliable, but we take no responsibility for the accuracy thereof.
Transfers of interests in one Global Note for interests in another Global Note
Prior to the expiration of the 40-Day Restricted Period, a Direct or an Indirect Participant who holds an interest in a Reg S Global Note will not be permitted to transfer its interest to a U.S. Person who takes delivery in the form of an interest in the Rule 144A Global Notes. After the expiration of the 40-Day Restricted Period, a Direct or an Indirect Participant who holds an interest in a Reg S Global Note will be permitted to transfer its interest to a U.S. Person who takes delivery in the form of an interest in the Rule 144A Global Notes only upon receipt by the Trustee of a written certification from the transferor to the effect that such transfer is being made in accordance with the restrictions on transfer set forth in the legend printed on the Reg S Global Notes.
Prior to the expiration of the 40-Day Restricted Period, a Direct or Indirect Participant who holds an interest in a Rule 144A Global Note will not be permitted to transfer its interests to any person that takes delivery thereof in the form of an interest in the Reg S Global Notes. After the expiration of the 40-Day Restricted Period, a Direct or Indirect Participant who holds an interest in a Rule 144A Global Note may transfer its interests to a person who takes delivery in the form of an interest in the Reg S Global Notes only upon receipt by the Trustee of a written certification from the transferor to the effect that such transfer is being made in accordance with Rule 904 of Regulation S.
Transfers involving an exchange of a beneficial interest in Reg S Global Notes for a beneficial interest in Rule 144A Global Notes or vice versa will be effected by DTC by means of an instruction originated by the Trustee through DTC's Deposit/Withdraw at Custodian (DWAC) system. Accordingly, in connection with such transfer, appropriate adjustments will be made to reflect a decrease in the
152
principal amount of the one Global Note and a corresponding increase in the principal amount of the other Global Note, as applicable. Any beneficial interest in the one Global Note that is transferred to a person who takes delivery in the form of the other Global Note will, upon transfer, cease to be an interest in such first Global Note and become an interest in such other Global Note and, accordingly, will thereafter be subject to all transfer restrictions and other procedures applicable to beneficial interests in such other Global Note for as long as it remains such an interest.
Transfers of interests in Global Notes for Certificated Notes
An entire Global Note may be exchanged for definitive notes in registered, certificated form without interest coupons ("Certificated Notes") if (i) DTC (x) notifies us that it is unwilling or unable to continue as depositary for the Global Notes and we thereupon fail to appoint a successor depositary within 90 days or (y) has ceased to be a clearing agency registered under the Exchange Act, (ii) we, at our option, notify the Trustee in writing that we elect to cause the issuance of Certificated Notes or (iii) upon the request of the Trustee or Holders of a majority of the outstanding principal amount of Outstanding Notes and New Notes, after there shall have occurred and be continuing a Default or an Event of Default with respect to the Outstanding Notes and New Notes. In any such case, we will notify the Trustee in writing that, upon surrender by the Direct and Indirect Participants of their interest in such Global Note, Certificated Notes will be issued to each person that such Direct and Indirect Participants and DTC identify as being the beneficial owner of the related notes.
Beneficial interests in Global Notes held by any Direct or Indirect Participant may be exchanged for Certificated Notes upon request to DTC, by such Direct Participant (for itself or on behalf of an Indirect Participant), to the Trustee in accordance with customary DTC procedures. Certificated Notes delivered in exchange for any beneficial interest in any Global Note will be registered in the names, and issued in any approved denominations, requested by DTC on behalf of such Direct or Indirect Participants (in accordance with DTC's customary procedures).
Neither we nor the Trustee will be liable for any delay by the holder of any Global Note or DTC in identifying the beneficial owners of Notes, and we and the Trustee may conclusively rely on, and will be protected in relying on, instructions from the holder of the Global Note or DTC for all purposes.
Same day settlement and payment
The Indenture will require that payments in respect of the Notes represented by the Global Notes (including principal, premium, if any, interest and Liquidated Damages, if any) be made by wire transfer of immediately available same day funds to the accounts specified by the holder of interests in such Global Note. With respect to Certificated Notes, we will make all payments of principal, premium, if any, interest and Liquidated Damages, if any, by wire transfer of immediately available same day funds to the accounts specified by the holders thereof or, if no such account is specified, by mailing a check to each such holder's registered address. We expect that secondary trading in the Certificated Notes will also be settled in immediately available funds.
153
UNITED STATES FEDERAL INCOME TAX CONSEQUENCES
The following discussion summarizes the material U.S. federal income tax consequences to beneficial owners arising from the exchange of Outstanding Notes for New Notes and the beneficial ownership and disposition of the New Notes. The discussion is based on provisions of the Code, its legislative history, judicial authority, current administrative rulings and practice, and existing and proposed Treasury Regulations, all as in effect and existing on the date hereof. Legislative, judicial or administrative changes or interpretations after the date hereof could alter or modify the validity of the statements and conclusions set forth below, may be retroactive and could adversely affect a beneficial owner of the New Notes.
The discussion that follows is intended as a descriptive summary only and is not intended as tax advice to any particular investor. This summary is not a complete analysis or listing of all potential U.S. federal income tax consequences and does not address the effect of any U.S. gift, estate, state or local tax law or any non-U.S. tax law. The actual tax consequences will vary depending upon the particular circumstances of each investor.
For purposes of this summary, a U.S. holder is a beneficial owner of the New Notes who is, for U.S. federal income tax purposes:
If a partnership (including an entity taxable as a partnership for U.S. federal income tax purposes) holds the Notes, the tax consequences of a partner will generally depend upon the status of the partner and upon the activities of the partnership. An investor who is a partner of a partnership holding the Notes should consult its own tax advisor. A "non-U.S. holder" is a beneficial owner of the Notes that is not a U.S. holder.
This summary is generally limited to investors who hold the Outstanding Notes and will hold the New Notes as "capital assets" within the meaning of Section 1221 of the Code and whose functional currency is the U.S. dollar. This summary does not address the tax treatment of investors that may be subject to special income and withholding tax rules including, without limitation:
Persons considering the exchange of their Outstanding Notes for New Notes are urged to consult their own tax advisers as to the United States or other tax consequences of the exchange of
154
Outstanding Notes for New Notes and the beneficial ownership and disposition of the New Notes, including the effect of any state, local or non-U.S. tax laws.
Taxation of U.S. Holders
Exchange Offer
A holder of Outstanding Notes will not recognize any taxable gain or loss on the exchange of an Outstanding Note for a New Note pursuant to the exchange offer, and such holder's tax basis and holding period in the New Note will be the same as in the Outstanding Note.
Payments of interest
You will be subject to tax on any interest on your New Note as ordinary income at the time you receive the interest or when it accrues, depending on your method of accounting for tax purposes. Although interest on the New Notes is currently expected to be treated as foreign source income for U.S. federal income tax purposes, it is possible that as a result of potential future restructurings, all or a portion of interest on the New Notes will be U.S. source income.
Market Discount
If a U.S. holder purchases a New Note (or purchased an Outstanding Note and exchanges it for a New Note) for an amount less than the stated principal amount of the New Note, the amount of such difference is "market discount" for federal income tax purposes, unless such difference is less than 1/4 of one percent of the stated principal amount multiplied by the number of complete years to maturity from the date of such purchase.
Unless such U.S. holder elects to include market discount in income as it accrues, any gain realized on the sale, exchange, retirement, or other disposition of a New Note and any partial principal payment received on a New Note generally will be treated as ordinary income to the extent of any accrued market discount on the New Note. In addition, a U.S. holder may be required to defer deductions for a portion of the interest paid on any indebtedness incurred to purchase or carry a New Note that has market discount.
In general, market discount on a New Note held by a U.S. holder will be considered to accrue ratably during the period from the date of purchase of the New Note to its maturity date, unless such U.S. holder elects to accrue market discount on a constant yield basis. U.S. holders may elect to include market discount in gross income currently as it accrues (on either a ratable or a constant yield basis), in which case the interest deferral rule described above will not apply. The election to include market discount in gross income on an accrual basis, once made, would apply to all market discount obligations acquired by the U.S. holder on or after the first day of the first taxable year to which the election applies, and it may not be revoked without the consent of the IRS. A U.S. holder's tax basis in the New Note will be increased by the amount of any market discount included in gross income under such an election. U.S. holders that hold New Notes with market discount should consult their tax advisors regarding the manner in which accrued market discount is calculated and the election to include market discount currently in income.
Bond Premium
In general, if a U.S. holder purchases a New Note (or purchased an Outstanding Note and exchanged it for a New Note) for an amount greater than the sum of all amounts payable on the New Note (other than stated interest payments) after the date of purchase, the amount of such excess is "bond premium" for United States federal income tax purposes. U.S. holders may elect to amortize bond premium over the remaining term of the New Note on a constant yield basis. The amount of bond premium allocable to any accrual period is offset against the stated interest allocable to the accrual period. A U.S. holder's tax basis in the New Note will be reduced by the amount of bond
155
premium so amortized. If a U.S. holder does not elect to amortize bond premium, it will be required to report the full amount of stated interest on the New Note as ordinary income, even though it may be required to recognize a capital loss (which may not be available to offset ordinary income) on a sale or other disposition of the New Note. An election to amortize bond premium, once made, would apply to all debt instruments held or subsequently acquired by the U.S. holder on or after the first day of the first taxable year to which the election applies, and may not be revoked without the consent of the IRS. U.S. holders that hold New Notes with bond premium should consult their tax advisors regarding the application of these rules.
Sale, exchange, retirement or other disposition of a New Note
Upon the sale, exchange, retirement or other disposition of a New Note, you will generally recognize gain or loss equal to the difference between the amount realized on such disposition and your tax basis in your New Note.
Gain or loss recognized on the sale, exchange, retirement or other disposition of a New Note generally will be capital gain or loss (except to the extent attributable to accrued but unpaid interest). Net capital gains derived with respect to capital assets held for more than one year are eligible for reduced rates of taxation. Certain limitations apply to the deductibility of capital losses.
Taxation of Non-U.S. Holders
Payments of interest
A non-U.S. holder will generally not be subject to U.S. federal income tax on payments of interest on the New Notes, provided that:
The certification requirement will be satisfied in any of the following circumstances:
If the requirements of the portfolio interest exemption described above are not satisfied, a 30% withholding tax may apply to the gross amount of interest on the New Notes that is paid to a non-U.S. holder, unless either: (a) an applicable income tax treaty reduces or eliminates such tax, and the non-U.S. holder claims the benefit of that treaty by providing a properly completed and duly executed IRS Form W-8BEN (or suitable successor or substitute form) establishing qualification for benefits
156
under the treaty, or (b) the interest is effectively connected with the non-U.S. holder's conduct of a trade or business in the United States and the non-U.S. holder provides an appropriate statement to that effect on a properly completed and duly executed IRS Form W-8ECI (or suitable successor form).
If a non-U.S. holder is engaged in a trade or business in the U.S. and interest on a New Note is effectively connected with the conduct of that trade or business, the non-U.S. holder will be required to pay U.S. federal income tax on that interest on a net income basis (and the 30% withholding tax described above will not apply provided the appropriate statement is provided to us) generally in the same manner as a U.S. person. If a non-U.S. holder is eligible for the benefits of an income tax treaty between the U.S. and its country of residence, any interest income that is effectively connected with a U.S. trade or business will be subject to U.S. federal income tax in the manner specified by the treaty and generally will only be subject to such tax if such income is attributable to a permanent establishment (or a fixed base in the case of an individual) maintained by the non-U.S. holder in the U.S. and the non-U.S. holder claims the benefit of the treaty by properly submitting an IRS Form W-8BEN. In addition, a non-U.S. holder that is treated as a foreign corporation for U.S. federal income tax purposes may be subject to a branch profits tax equal to 30% (or lower applicable treaty rate) of its earnings and profits for the taxable year, subject to adjustments, that are effectively connected with its conduct of a trade or business in the U.S.
Sale, exchange, retirement or other disposition of the New Notes.
Subject to the discussion of backup withholding below, a non-U.S. holder generally will not be subject to U.S. federal income tax (or any withholding thereof) on any gain realized by such holder upon a sale, exchange, redemption, retirement at maturity or other disposition of a New Note, unless:
If the first exception applies, the non-U.S. holder generally will be subject to U.S. federal income tax at a rate of 30% on the amount by which its U.S.-source capital gains exceed its U.S.-source capital losses. If the second exception applies, the non-U.S. holder will generally be subject to U.S. federal income tax on the net gain derived from the sale, exchange or other disposition of the New Notes in the same manner as a U.S. person. In addition, corporate non-U.S. holders may be subject to a 30% branch profits tax on effectively connected gain. If a non-U.S. holder is eligible for the benefits of an income tax treaty between the United States and its country of residence, the U.S. federal income tax treatment of any such gain may be modified in the manner specified by the treaty.
Backup Withholding and Information Reporting
In general, if you are a non-corporate U.S. holder, the payors of interest and principal on the New Notes will be required to report to the IRS all such payments on your New Note. In addition, payors are required to report to the IRS any payment of proceeds of the sale of your New Note before maturity within the United States. Backup withholding will also apply to any such payments, if you fail to provide an accurate taxpayer identification number, or you are notified by the IRS that you have failed to report all interest and dividends required to be shown on your U.S. federal income tax returns.
Non-U.S. holders may be required to comply with certification procedures to establish that the holder is not a U.S. person in order to avoid backup withholding tax and information reporting requirements discussed above.
157
If you intend to use plan assets to exchange for any of the New Notes offered by this prospectus, you should consult with counsel on the potential consequences of your investment under the fiduciary responsibility and prohibited transaction provisions of the Employee Retirement Income Security Act of 1974, as amended ("ERISA"), and the prohibited transaction provisions of the Code. If you intend to use governmental or church plan assets to exchange for any of the New Notes, you should consult with counsel on the potential consequences of your investment under similar provisions applicable under laws governing governmental and church plans.
The following summary is based on the provisions of ERISA and the Code and related guidance in effect as of the date of this prospectus. This summary does not attempt to be a complete summary of these considerations. Future legislation, court decisions, administrative regulations or other guidance will change the requirements summarized in this section. Any of these changes could be made retroactively and could apply to transactions entered into before the change is enacted.
Fiduciary Responsibilities
ERISA imposes requirements on (1) employee benefit plans subject to ERISA, (2) entities whose underlying assets include employee benefit plan assets, for example, collective investment funds and insurance company general accounts, and (3) fiduciaries of employee benefit plans. Under ERISA, fiduciaries generally include persons who exercise discretionary authority or control over plan assets. Before using any employee benefit plan assets to exchange for any of the New Notes offered in connection with this prospectus, you should determine whether the investment:
(1) is permitted under the plan document and other instruments governing the plan; and
(2) is appropriate for the plan in view of its overall investment policy and the composition and diversification of its portfolio, taking into account the limited liquidity of the New Notes.
You should consider all factors and circumstances of a particular investment in the New Notes, including, for example, the matters set forth under the heading "Risk Factors" and the fact that in the future there may not be a market in which you will be able to sell or otherwise dispose of your interest in the New Notes.
We are not making any representation that the exchange for New Notes under a plan meets the fiduciary requirements for investment by plans generally or any particular plan or that such an investment is appropriate for plans generally or any particular plan.
Prohibited Transactions
ERISA and the Code prohibit a wide range of transactions involving (1) employee benefit plans and arrangements subject to ERISA and/or the Code, and (2) persons who have specified relationships to the plans. These persons are called "parties in interest" under ERISA and "disqualified persons" under the Code. The transactions prohibited by ERISA and the Code are called "prohibited transactions." If you are a party in interest or disqualified person who engages in a prohibited transaction, you may be subject to excise taxes and other penalties and liabilities under ERISA and/or the Code. As a result, if you are considering using plan assets to exchange for any of the New Notes offered by this prospectus, you should consider whether the investment might be a prohibited transaction under ERISA and/or the Code.
Prohibited transactions may arise, for example, if the exchange under a plan with respect to which we, or any of our affiliates, are a party in interest or a disqualified person. Exemptions from the prohibited transaction provisions of ERISA and the Code may apply depending in part on the type of plan fiduciary making the decision to exchange and the circumstances under which such decision is made. Some of these exemptions include:
158
(1) Prohibited transaction class exemption or "PTCE" exemptions 75-1 (relating to specified transactions involving employee benefit plans and broker-dealers, reporting dealers and banks);
(2) PTCE 84-14 (relating to specified transactions directed by independent qualified professional asset managers);
(3) PTCE 90-1 (relating to specified transactions involving insurance company pooled separate accounts);
(4) PTCE 91-38 (relating to specified transactions by bank collective investment funds);
(5) PTCE 95-60 (relating to specified transactions involving insurance company general accounts); and
(6) PTCE 96-23 (relating to specified transactions directed by in-house asset managers).
These exemptions do not, however, provide relief from the self-dealing prohibitions under ERISA and the Code. In addition, there is no assurance that any of these class exemptions or other exemption will be available with respect to any particular transaction involving the exchange.
Government and Church Plans
Governmental plans and some church plans, while not subject to the fiduciary responsibility provisions of ERISA or the prohibited transactions provisions of ERISA or the Code, may be subject to state or other federal laws that are very similar to the provisions of ERISA and the Code. If you are a fiduciary of a governmental or church plan, you should consult with counsel before exchanging for any of the New Notes offered by this prospectus.
Foreign Indicia of Ownership
ERISA also prohibits plan fiduciaries from maintaining the indicia of ownership of any plan assets outside the jurisdiction of the United States district courts except in specified cases. Before exchanging for any of the New Notes offered by this prospectus, you should consider whether the exchange, acquisition, holding or disposition of the New Notes would satisfy such indicia of ownership rules.
Representations and Warranties
If you acquire, exchange for, or accept New Notes offered by this prospectus, you will be deemed to have represented and warranted that either:
(1) you have not used plan assets to acquire, or exchange for, such New Note; or
(2) your acquisition, or exchange for, and holding of a note (A) is exempt from the prohibited transaction restrictions of ERISA and the Code under one or more prohibited transaction class exemptions or does not constitute a prohibited transaction under ERISA and the Code, and (B) meets the fiduciary requirements of ERISA.
159
CAYMAN ISLANDS TAX CONSEQUENCES
The following is a discussion of certain Cayman Islands income tax consequences of an investment in the Notes. The discussion is a general summary of present law, which is subject to prospective and retroactive change. It is not intended as tax advice, does not consider any investor's particular circumstances, and does not consider tax consequences other than those arising under Cayman Islands law.
Under existing Cayman Islands Laws:
The Company has been incorporated under the laws of the Cayman Islands as an exempted company and, as such, has obtained an undertaking from the Governor in Cabinet of the Cayman Islands in the following form:
The Tax Concessions Law
1999 Revision
Undertaking as to Tax Concessions
In accordance with the provision of Section 6 of The Tax Concession Law (1999 Revision), the Governor in Cabinet undertakes with WH Holdings (Cayman Islands) Ltd. "the Company".
These concessions shall be for a period of twenty years from the 16th day of April, 2002.
160
Each broker-dealer that receives Exchange Securities for its own account pursuant to the Exchange Offer must acknowledge that it will deliver a prospectus in connection with any resale of such Exchange Securities. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of New Notes received in exchange for Outstanding Notes where such Outstanding Notes were acquired as a result of market-making activities or other trading activities. We have agreed that, for a period of 180 days after the Expiration Date, we will make this prospectus, as amended or supplemented, available to any broker-dealer for use in connection with any such resale.
We will not receive any proceeds from any sale of New Notes by broker-dealers. New Notes received by broker-dealers for their own account pursuant to the exchange offer may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the New Notes or a combination of such methods of resale, at market prices prevailing at the time of resale, at prices related to such prevailing market prices or negotiated prices. Any such resale may be made directly to purchasers or to or through brokers or dealers who may receive compensation in the form of commissions or concessions from any such broker-dealer or the purchasers of any such New Notes. Any broker-dealer that resells New Notes that were received by it for its own account pursuant to the exchange offer and any broker or dealer that participates in a distribution of such New Notes may be deemed to be an "underwriter" within the meaning of the Securities Act and any profit on any such resale of New Notes and any commission or concessions received by any such persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that, by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an "underwriter" within the meaning of the Securities Act.
For a period of 180 days after the Expiration Date we will promptly send additional copies of this prospectus and any amendment or supplement to this prospectus to any broker-dealer that requests such documents in the letter of transmittal. We have agreed to pay all expenses incident to the exchange offer other than commissions or concessions of any brokers or dealers and will indemnify original holders of the New Notes (including any broker-dealers) against certain liabilities, including liabilities under the Securities Act.
We have not entered into any arrangements or understanding with any person to distribute the New Notes to be received in the exchange offer.
161
The validity of the Notes offered hereby and certain legal matters in connection with this offering will be passed upon for Holdings and for WH Capital Corporation by Gibson, Dunn & Crutcher LLP, Los Angeles, California. Certain legal matters as to matters of Cayman Islands law in connection with the offering will also be passed upon for Holdings by Maples and Calder, Grand Cayman, Cayman Islands. Certain legal matters as to matters of Nevada law in connection with the offering will be passed upon for WH Capital Corporation by Schreck Brignone. Certain legal matters in connection with this offering will be passed upon for the initial purchaser by Skadden, Arps, Slate, Meagher & Flom LLP, Los Angeles, California.
The consolidated financial statements of WH Holdings (Cayman Islands) Ltd. as of December 31, 2003, and for the year then ended, have been included herein in reliance upon the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
The consolidated financial statements of Herbalife International, Inc., the predecessor, for the year ended December 31, 2001 and the seven months ended July 31, 2002 and WH Holdings (Cayman Islands) Ltd., the successor, as of December 31, 2002 and for the five months ended December 31, 2002, included in this prospectus, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein, and have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
We may not be subject to the periodic reporting and other information requirements of the Exchange Act. Pursuant to the indenture governing the Outstanding Notes and the New Notes, we agreed that, for so long as any of the Notes remains outstanding, we will furnish to the holders of the Notes (i) all annual and quarterly financial information that would be required to be contained in a filing with the Commission on Forms 10-K and 10-Q if we were required to file such forms, including for each such form, a "management's discussion and analysis of financial condition and results of operations" and, with respect to the annual financial information only, a report thereon by our certified independent auditors and (ii) all current reports that would be required to be furnished to the Commission on Form 8-K if we were required to furnish such reports. Furthermore, for so long as any of the Outstanding Notes remain outstanding, we have agreed to make available to the holders of the Outstanding Notes and any prospective investor, upon request of such holders, the information required to be delivered pursuant to Rule 144(a)(d)(4) under the Securities Act so long as the Outstanding Notes are "restricted securities" within the meaning of Rule 144(a)(3) under the Securities Act.
162
WH HOLDINGS (CAYMAN ISLANDS) LTD.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| |
Page |
|
|---|---|---|
| Report of Independent Registered Public Accounting Firm KPMG, LLP | F-2 | |
Report of Independent Registered Public Accounting Firm Deloitte & Touche LLP |
F-3 |
|
Consolidated Balance Sheets as of December 31, 2002, and December 31, 2003 |
F-4 |
|
Consolidated Statements of Income for the years ended December 31, 2001, 2002 and 2003 |
F-5 |
|
Consolidated Statements of Changes in Shareholders' Equity for the years ended December 31, 2001, 2002 and 2003 |
F-6 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2001, 2002 and 2003 |
F-7 |
|
Notes to Consolidated Financial Statements for the years ended December 31, 2001, 2002, and 2003 |
F-8 |
|
Consolidated Balance Sheets as of December 31, 2003 and March 31, 2004 (unaudited) |
F-44 |
|
Consolidated Statements of Income for the three months ended March 31, 2003 and March 31, 2004 (unaudited) |
F-45 |
|
Consolidated Statements of Cash Flows for the three months ended March 31, 2003 and March 31, 2004 (unaudited) |
F-46 |
|
Notes to Consolidated Financial Statements for the three months ended March 31, 2003 and March 31, 2004 (unaudited) |
F-47 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of WH Holdings (Cayman Islands) Ltd:
We have audited the accompanying consolidated balance sheet of WH Holdings (Cayman Islands) Ltd. and subsidiaries as of December 31, 2003 and the related consolidated statements of income, changes in shareholders' equity, and cash flows for the year ended December 31, 2003. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of WH Holdings (Cayman Islands) Ltd. and subsidiaries as of December 31, 2003, and the results of their operations and their cash flows for the year ended December 31, 2003, in conformity with U.S. generally accepted accounting principles.

Los
Angeles, California
February 19, 2004,
except as to Note 17 which is as of March 8, 2004
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of WH Holdings (Cayman Islands) Ltd:
We have audited the accompanying consolidated balance sheet of WH Holdings (Cayman Islands) Ltd. and subsidiaries (the "Successor") as of December 31, 2002, and the related consolidated statements of income, changes in shareholders' equity, and cash flows for the five-month period ended December 31, 2002. We have also audited the related consolidated statements of income, changes in shareholders' equity, and cash flows of Herbalife International, Inc. and subsidiaries (the "Predecessor"), a wholly owned subsidiary of the Successor, for the seven-month period ended July 31, 2002 and the year ended December 31, 2001. These financial statements are the responsibility of Successor and Predecessor management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of the Successor as of December 31, 2002, and the results of its operations and its cash flows for the five-month period ended December 31, 2002, and the results of operations of the Predecessor and its cash flows for the seven-month period ended July 31, 2002, and the year ended December 31, 2001, in conformity with accounting principles generally accepted in the United States of America.
![]()
DELOITTE & TOUCHE LLP
Los
Angeles, California
February 19, 2004
F-3
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED BALANCE SHEETS
(as of December 31)
| |
2002 |
2003 |
||||||
|---|---|---|---|---|---|---|---|---|
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 64,201,000 | $ | 150,679,000 | ||||
| Restricted cash | 10,551,000 | 5,701,000 | ||||||
| Marketable securities | 1,272,000 | — | ||||||
| Receivables, net of allowance for doubtful accounts of $2,527,000 (2003) and $3,257,000 (2002), including related party receivables of $323,000 (2003) and $506,000 (2002) | 29,026,000 | 31,977,000 | ||||||
| Inventories | 56,868,000 | 59,397,000 | ||||||
| Prepaid expenses and other current assets | 16,081,000 | 20,825,000 | ||||||
| Deferred income taxes | 26,705,000 | 9,164,000 | ||||||
| Total current assets | 204,704,000 | 277,743,000 | ||||||
Property—at cost: |
||||||||
| Furniture and fixtures | 5,144,000 | 6,137,000 | ||||||
| Equipment | 41,598,000 | 48,148,000 | ||||||
| Leasehold improvements | 7,045,000 | 8,733,000 | ||||||
| 53,787,000 | 63,018,000 | |||||||
| Less: accumulated depreciation and amortization | (7,675,000 | ) | (17,607,000 | ) | ||||
| Net property | 46,112,000 | 45,411,000 | ||||||
Deferred compensation assets |
31,922,000 |
21,340,000 |
||||||
| Other assets | 5,327,000 | 5,795,000 | ||||||
| Deferred financing costs, net of accumulated amortization of $10,266,000 (2003) and $3,564,000 (2002) | 40,719,000 | 33,278,000 | ||||||
| Marketing related intangibles | 310,000,000 | 310,000,000 | ||||||
| Distributor network, net of accumulated amortization of $26,539,000 (2003) | — | 29,661,000 | ||||||
| Product certifications, product formulas and other intangible assets, net of accumulated amortization of $9,491,000 (2003) and $1,542,000 (2002) | 5,858,000 | 13,219,000 | ||||||
| Goodwill | 211,063,000 | 167,517,000 | ||||||
| TOTAL | $ | 855,705,000 | $ | 903,964,000 | ||||
LIABILITIES AND SHAREHOLDERS' EQUITY |
||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 21,580,000 | $ | 22,526,000 | ||||
| Royalty overrides | 69,062,000 | 76,522,000 | ||||||
| Accrued compensation | 22,443,000 | 19,127,000 | ||||||
| Accrued expenses | 47,341,000 | 59,669,000 | ||||||
| Current portion of long term debt | 19,160,000 | 72,377,000 | ||||||
| Advance sales deposits | 6,306,000 | 6,574,000 | ||||||
| Income taxes payable | 11,626,000 | 19,427,000 | ||||||
| Total current liabilities | 197,518,000 | 276,222,000 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Long-term debt, net of current portion, including related party debt of $23.7 million (2003) and $23.2 million (2002) | 321,599,000 | 252,917,000 | ||||||
| Deferred compensation liability | 32,082,000 | 22,442,000 | ||||||
| Deferred income taxes | 110,707,000 | 111,910,000 | ||||||
| Other non-current liabilities | 2,525,000 | 2,685,000 | ||||||
| Total liabilities | 664,431,000 | 666,176,000 | ||||||
SHAREHOLDERS' EQUITY: |
||||||||
| Preferred shares, $0.001 par value (aggregate liquidation preference $446,241,000 (2003), and $291,291,000 (2002)), 12% Series A Cumulative and Convertible, 106,000,000 (2003) and 103,000,000 (2002) shares authorized, 102,013,572 (2003) and 100,000,000 (2002) shares issued and outstanding | 100,000 | 102,000 | ||||||
| Common shares, $0.001 par value, 250,000,000 shares authorized, no shares issued and outstanding | — | — | ||||||
| Paid-in capital in excess of par value | 177,308,000 | 183,407,000 | ||||||
| Accumulated other comprehensive income (loss) | (139,000 | ) | 3,427,000 | |||||
| Retained earnings | 14,005,000 | 50,852,000 | ||||||
| Total shareholders' equity | 191,274,000 | 237,788,000 | ||||||
| TOTAL | $ | 855,705,000 | $ | 903,964,000 | ||||
See the accompanying Notes to Consolidated Financial Statements.
F-4
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED STATEMENTS OF INCOME
| |
2001 |
2002 |
2003 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, |
January 1 to July 31 |
August 1 to December 31 |
Year ended December 31, |
||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
||||||||
| Product sales | $ | 881,655,000 | $ | 554,693,000 | $ | 386,360,000 | $ | 995,120,000 | ||||
| Handling & freight income | 138,475,000 | 89,495,000 | 63,164,000 | 164,313,000 | ||||||||
| Net sales | 1,020,130,000 | 644,188,000 | 449,524,000 | 1,159,433,000 | ||||||||
| Cost of sales | 241,522,000 | 140,553,000 | 95,001,000 | 235,785,000 | ||||||||
Gross profit |
778,608,000 |
503,635,000 |
354,523,000 |
923,648,000 |
||||||||
| Royalty overrides | 355,225,000 | 227,233,000 | 159,915,000 | 415,351,000 | ||||||||
| Marketing, distribution & administrative expenses, including $8,400,000 (2003) and $2,200,000 (period from August 1 to December 31, 2002) of related party expenses | 354,608,000 | 207,390,000 | 135,536,000 | 401,261,000 | ||||||||
| Merger transaction expenses | — | 54,708,000 | 6,183,000 | — | ||||||||
| Interest expense (income)—net | (3,413,000 | ) | (1,364,000 | ) | 23,898,000 | 41,468,000 | ||||||
| Income before income taxes and minority interest | 72,188,000 | 15,668,000 | 28,991,000 | 65,568,000 | ||||||||
| Income taxes | 28,875,000 | 6,267,000 | 14,986,000 | 28,721,000 | ||||||||
| Net income before minority interest | 43,313,000 | 9,401,000 | 14,005,000 | 36,847,000 | ||||||||
| Minority interest | 725,000 | 189,000 | — | — | ||||||||
| NET INCOME | $ | 42,588,000 | $ | 9,212,000 | $ | 14,005,000 | $ | 36,847,000 | ||||
See the accompanying Notes to Consolidated Financial Statements.
F-5
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS' EQUITY
| Predecessor |
Common Stock A |
Common Stock B |
Paid in Capital in Excess of Par Value |
Accumulated Other Comprehensive Income (Loss) |
Retained Earnings |
Total Shareholders' Equity |
Comprehensive Income |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balance at December 31, 2000 | $ | 102,000 | $ | 190,000 | $ | 58,860,000 | $ | (7,010,000 | ) | $ | 170,259,000 | $ | 222,401,000 | |||||||||
| Issuance of 1,061,859 shares of Class A Common Stock and 1,298,965 Shares of Class B Common Stock under 1991 Stock Option Plan and other | 10,000 | 13,000 | 17,434,000 | 10,000 | 17,467,000 | |||||||||||||||||
| Additional capital from tax benefit of 1991 stock option plan | 1,423,000 | 1,423,000 | ||||||||||||||||||||
| Net income | 42,588,000 | 42,588,000 | $ | 42,588,000 | ||||||||||||||||||
| Translation adjustments | (6,817,000 | ) | (6,817,000 | ) | (6,817,000 | ) | ||||||||||||||||
| Unrealized gain on marketable securities | 12,000 | 12,000 | 12,000 | |||||||||||||||||||
| Cumulative effect on accounting change | 909,000 | 909,000 | 909,000 | |||||||||||||||||||
| Unrealized gain (loss) on derivatives | 4,815,000 | 4,815,000 | 4,815,000 | |||||||||||||||||||
| Reclassification adjustments for gain (loss) on derivative instruments | (3,440,000 | ) | (3,440,000 | ) | (3,440,000 | ) | ||||||||||||||||
| Total comprehensive income | — | $ | 38,067,000 | |||||||||||||||||||
| Cash dividends declared | (18,442,000 | ) | (18,442,000 | ) | ||||||||||||||||||
| Balance at December 31, 2001 | $ | 112,000 | $ | 203,000 | $ | 77,717,000 | $ | (11,531,000 | ) | $ | 194,415,000 | $ | 260,916,000 | |||||||||
| Issuance of 346,695 shares of Class A Common Stock and 1,139,237 Shares of Class B Common Stock under the 1991 Stock Option Plan and other | 4,000 | 11,000 | 10,531,000 | 10,546,000 | ||||||||||||||||||
| Additional capital from revaluation of stock options | 980,000 | 980,000 | ||||||||||||||||||||
| Additional capital from tax benefit of 1991 stock option plan | 3,042,000 | 3,042,000 | ||||||||||||||||||||
| Other | 375,000 | 375,000 | ||||||||||||||||||||
| Net income | 9,212,000 | 9,212,000 | $ | 9,212,000 | ||||||||||||||||||
| Translation adjustments | 1,428,000 | 1,428,000 | 1,428,000 | |||||||||||||||||||
| Unrealized gain on marketable securities | 14,000 | 14,000 | 14,000 | |||||||||||||||||||
| Unrealized gain (loss) on derivatives | (3,338,000 | ) | (3,338,000 | ) | (3,338,000 | ) | ||||||||||||||||
| Reclassification adjustments for gain (loss) on derivative instruments | 1,315,000 | 1,315,000 | 1,315,000 | |||||||||||||||||||
| Total comprehensive income | — | $ | 8,631,000 | |||||||||||||||||||
| Cash dividends declared | (4,962,000 | ) | (4,962,000 | ) | ||||||||||||||||||
| Balance at July 31, 2002 | $ | 116,000 | $ | 214,000 | $ | 92,645,000 | $ | (12,112,000 | ) | $ | 198,665,000 | $ | 279,528,000 | |||||||||
| Successor |
Preferred Stock |
Paid in Capital in Excess of Par Value |
Accumulated Other Comprehensive Income (Loss) |
Retained Earnings |
Total Shareholders' Equity |
Comprehensive Income |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Issuance of 100,000,000 Preferred Shares | $ | 100,000 | $ | 175,508,000 | $ | 175,608,000 | |||||||||||||
| Issuance of stock warrants (Note 4) | 1,800,000 | 1,800,000 | |||||||||||||||||
| Net income | $ | 14,005,000 | 14,005,000 | $ | 14,005,000 | ||||||||||||||
| Translation adjustments | $ | 302,000 | 302,000 | 302,000 | |||||||||||||||
| Unrealized gain on marketable securities | 4,000 | 4,000 | 4,000 | ||||||||||||||||
| Unrealized gain (loss) on derivatives | 2,266,000 | 2,266,000 | 2,266,000 | ||||||||||||||||
| Reclassification adjustments for gain (loss) on derivative instruments | (2,711,000 | ) | (2,711,000 | ) | (2,711,000 | ) | |||||||||||||
| Total comprehensive income | $ | 13,866,000 | |||||||||||||||||
| Balance at December 31, 2002 | $ | 100,000 | $ | 177,308,000 | $ | (139,000 | ) | $ | 14,005,000 | $ | 191,274,000 | ||||||||
| Issuance of 2,013,572 Preferred Shares | 2,000 | 4,204,000 | 4,206,000 | ||||||||||||||||
| Stock options | 1,895,000 | 1,895,000 | |||||||||||||||||
| Net income | 36,847,000 | 36,847,000 | $ | 36,847,000 | |||||||||||||||
| Translation adjustments | 4,517,000 | 4,517,000 | 4,517,000 | ||||||||||||||||
| Unrealized gain on marketable securities | (4,000 | ) | (4,000 | ) | (4,000 | ) | |||||||||||||
| Unrealized gain (loss) on derivatives | (464,000 | ) | (464,000 | ) | (464,000 | ) | |||||||||||||
| Reclassification adjustments for gain (loss) on derivative instruments | (483,000 | ) | (483,000 | ) | (483,000 | ) | |||||||||||||
| Total comprehensive income | $ | 40,413,000 | |||||||||||||||||
| Balance at December 31, 2003 | $ | 102,000 | $ | 183,407,000 | $ | 3,427,000 | $ | 50,852,000 | $ | 237,788,000 | |||||||||
See the accompanying Notes to Consolidated Financial Statements.
F-6
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
2001 |
2002 |
2003 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, |
January 1 to July 31 |
August 1 to December 31 |
Year ended December 31, |
|||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
|||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | |||||||||||||
| Net income | $ | 42,588,000 | $ | 9,212,000 | $ | 14,005,000 | $ | 36,847,000 | |||||
Adjustments to reconcile net income to net cash provided by (used in) operating activities: |
|||||||||||||
| Depreciation and amortization | 18,056,000 | 11,722,000 | 11,424,000 | 55,605,000 | |||||||||
| Amortization of discount and deferred financing costs | — | — | 3,651,000 | 7,039,000 | |||||||||
| Deferred income taxes | (3,036,000 | ) | 3,186,000 | (16,981,000 | ) | (12,160,000 | ) | ||||||
| Unrealized foreign exchange loss | 383,000 | 2,448,000 | 433,000 | 4,070,000 | |||||||||
| Loss on repurchase of senior subordinated notes | — | — | — | 1,368,000 | |||||||||
| Minority interest in earnings | 725,000 | 189,000 | — | — | |||||||||
| Other | 515,000 | 2,338,000 | (719,000 | ) | 3,072,000 | ||||||||
| Changes in operating assets and liabilities: | |||||||||||||
| Receivables | (3,867,000 | ) | (11,712,000 | ) | 11,408,000 | (481,000 | ) | ||||||
| Inventories | 24,154,000 | 11,462,000 | 3,576,000 | 592,000 | |||||||||
| Prepaid expenses and other current assets | (5,542,000 | ) | (14,107,000 | ) | 9,972,000 | (4,188,000 | ) | ||||||
| Accounts payable | 2,135,000 | 14,831,000 | (12,132,000 | ) | (821,000 | ) | |||||||
| Royalty overrides | (8,206,000 | ) | 3,948,000 | 3,940,000 | 1,526,000 | ||||||||
| Accrued expenses and accrued compensation | 15,557,000 | 1,895,000 | (7,611,000 | ) | 5,045,000 | ||||||||
| Advance sales deposits | (163,000 | ) | 3,230,000 | (3,277,000 | ) | (454,000 | ) | ||||||
| Income taxes payable | 5,452,000 | 718,000 | 11,476,000 | 7,228,000 | |||||||||
| Deferred compensation liability | 6,714,000 | (1,459,000 | ) | (1,126,000 | ) | (9,640,000 | ) | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | 95,465,000 | 37,901,000 | 28,039,000 | 94,648,000 | |||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | |||||||||||||
| Purchases of property | (10,940,000 | ) | (4,741,000 | ) | (2,190,000 | ) | (13,601,000 | ) | |||||
| Proceeds from sale of property | 145,000 | 191,000 | 46,000 | 53,000 | |||||||||
| Net change in restricted cash | — | — | (10,551,000 | ) | 4,850,000 | ||||||||
| Net changes in marketable securities | 7,981,000 | 20,691,000 | (2,000 | ) | 1,268,000 | ||||||||
| Other assets | (1,644,000 | ) | (2,300,000 | ) | (421,000 | ) | (298,000 | ) | |||||
| Deferred compensation assets | (11,908,000 | ) | 5,154,000 | 6,145,000 | 10,582,000 | ||||||||
Acquisition of Herbalife International, Inc. (net of cash acquired of $201,821,000) |
— |
— |
(449,073,000 |
) |
— |
||||||||
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | (16,366,000 | ) | 18,995,000 | (456,046,000 | ) | 2,854,000 | |||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | |||||||||||||
| Dividends paid | (18,094,000 | ) | (9,682,000 | ) | — | — | |||||||
| Distribution to minority interest | (1,272,000 | ) | (4,598,000 | ) | — | — | |||||||
| Borrowings from long-term debt | 1,903,000 | 29,000 | 383,199,000 | 6,508,000 | |||||||||
| Principal payments on long-term debt | (3,460,000 | ) | (3,799,000 | ) | (51,069,000 | ) | (23,864,000 | ) | |||||
| Repurchase of senior subordinated notes | — | — | — | (5,681,000 | ) | ||||||||
| Increase in deferred financing costs | — | (27,788,000 | ) | (16,219,000 | ) | — | |||||||
| Exercise of stock options | 17,467,000 | 10,546,000 | — | — | |||||||||
| Issuance of preferred stock | — | — | 175,608,000 | 4,206,000 | |||||||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | (3,456,000 | ) | (35,292,000 | ) | 491,519,000 | (18,831,000 | ) | ||||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH | (6,742,000 | ) | 980,000 | 689,000 | 7,807,000 | ||||||||
| NET CHANGE IN CASH AND CASH EQUIVALENTS | 68,901,000 | 22,584,000 | 64,201,000 | 86,478,000 | |||||||||
| CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | 110,336,000 | 179,237,000 | — | 64,201,000 | |||||||||
| CASH AND CASH EQUIVALENTS, END OF PERIOD | $ | 179,237,000 | $ | 201,821,000 | $ | 64,201,000 | $ | 150,679,000 | |||||
| CASH PAID DURING THE YEAR | |||||||||||||
| Interest paid | $ | 1,079,000 | $ | 287,000 | $ | 5,814,000 | $ | 35,866,000 | |||||
| Income taxes paid | $ | 28,693,000 | $ | 16,479,000 | $ | 10,986,000 | $ | 32,836,000 | |||||
| NON CASH ACTIVITIES | |||||||||||||
| Acquisitions of property from capital leases | $ | 3,811,000 | $ | 2,058,000 | $ | 1,409,000 | $ | 6,834,000 | |||||
See the accompanying Notes to Consolidated Financial Statements.
F-7
WH HOLDINGS (CAYMAN ISLANDS) LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization
WH Holdings (Cayman Islands) Ltd., a Cayman Islands exempted limited liability company ("Holdings"), incorporated on April 4, 2002, and its direct and indirect wholly owned subsidiaries, WH Intermediate Holdings Ltd., a Cayman Islands company ("WH Intermediate"), WH Luxembourg Holdings S.à.R.L., a Luxembourg unipersonal limited liability company ("Lux Holdings"), WH Luxembourg Intermediate Holdings S.à.R.L., a Luxembourg unipersonal limited liability company ("Lux Intermediate"), Herbalife International Luxembourg S.à.R.L. ("Herbalife Lux"), formerly known as WH Luxembourg CM S.à.R.L., a Luxembourg unipersonal limited liability company, and WH Acquisition Corp., a Nevada corporation ("WH Acquisition"), were formed on behalf of Whitney & Co., LLC ("Whitney") and Golden Gate Private Equity, Inc. ("Golden Gate"), in order to acquire Herbalife International, Inc., a Nevada corporation, and its subsidiaries ("Herbalife" or "Predecessor") on July 31, 2002 (the "Merger"). Holdings and its subsidiaries are referred to collectively herein as the Company. Holdings' 12% Series A Cumulative Convertible Preferred Shares are referred to as "preferred shares" and Holdings' Common Shares are referred to as "common shares."
On July 31, 2002, WH Acquisition merged with and into Herbalife with Herbalife being the surviving corporation. The Merger was consummated pursuant to the Agreement and Plan of Merger by and among the Company, sole shareholder of WH Intermediate and a Cayman Islands company, WH Acquisition and Herbalife entered into on April 10, 2002 (the "Merger Agreement"). Each shareholder of Herbalife received $19.50 in cash for each common share. The holders of each outstanding option to purchase Herbalife common shares received an amount in cash equal to the excess of $19.50 over the exercise price of such option. As a result of the Merger, Herbalife was delisted from the NASDAQ National Market. The shares of Herbalife are no longer publicly traded and, therefore, earnings per share calculations are no longer included for financial statement presentations.
The Merger has been accounted for as a purchase in accordance with Statement of Financial Accounting Standards No. ("SFAS") 141, "Business Combinations." Accordingly, the acquired assets and liabilities have been recorded at fair value. Because of this, different bases of accounting have been used to prepare the Company and Predecessor consolidated financial statements. In the future, the primary differences are expected to relate to additional interest expense on the new debt, amortization of intangibles, and amortization of deferred financing costs recorded at the date of the Merger.
The Company completed the final allocation of the purchase price in connection with the Merger during 2003 based on an independent valuation study. The study was used as the basis to make the final determination of the values that should be allocated to various finite and indefinite lived intangible assets as well as goodwill. As a result of this completion of the purchase price allocation process, certain reclassifications were made to certain categories of intangible assets and goodwill that were previously identified on a preliminary basis as of December 31, 2002.
The total purchase price of approximately $651.5 million was allocated to the acquired assets and assumed liabilities based upon their respective fair value as of the closing date using valuations and
F-8
other studies that have been finalized. The following table summarizes the fair values of the assets acquired and the liabilities assumed at the date of Acquisition:
| |
Final Allocation |
Preliminary Allocation |
Increase (Decrease) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(dollars in millions) |
|||||||||
| Current assets | $ | 388.7 | $ | 388.7 | $ | — | ||||
| Property | 52.0 | 52.0 | — | |||||||
| Marketing related intangibles | 310.0 | 310.0 | — | |||||||
| Distributor network | 56.2 | — | 56.2 | |||||||
| Product formulas | 15.5 | — | 15.5 | |||||||
| Product certifications and other intangible assets | 7.2 | 7.4 | (0.2 | ) | ||||||
| Goodwill | 167.5 | 211.1 | (43.6 | ) | ||||||
| Other long-term assets | 42.6 | 42.6 | — | |||||||
| Total assets acquired | $ | 1,039.7 | $ | 1,011.8 | $ | 27.9 | ||||
Current liabilities |
$ |
209.4 |
$ |
209.4 |
$ |
— |
||||
| Other non-current liabilities | 34.9 | 34.9 | — | |||||||
| Long-term debt | 1.2 | 1.2 | — | |||||||
| Deferred income taxes | 142.7 | 114.8 | 27.9 | |||||||
| Total liabilities assumed | $ | 388.2 | $ | 360.3 | $ | 27.9 | ||||
| Net assets acquired | $ | 651.5 | $ | 651.5 | $ | — | ||||
Marketing related intangibles are considered to have an indefinite life and are not subject to amortization. Distributor network has an expected life of three years. Product formulas have an expected life of five years. Product certifications have an expected life of two years. None of the intangibles are expected to be deductible for tax purposes. As a result of the finalization of the purchase price allocation during the third quarter of 2003, the Company recorded additional amortization expense of $19.1 million before tax relating to periods prior to July 1, 2003. The Company recorded total amortization expense of $34.5 million before tax for 2003 and $1.5 million for the period from August 1 to December 31, 2002. In addition, the amounts for marketing franchise, trademark and trade name as of December 31, 2002 have been combined and are presented as marketing related intangibles above and in the accompanying balance sheet to conform to the current year presentation.
In connection with the Merger, the Predecessor incurred transaction expenses and stock option payments of approximately $54.7 million, which have been reflected in the Predecessor financial statements. The Company also incurred transaction expenses of approximately $6.2 million. In addition, the Company incurred debt issuance costs of approximately $44.3 million, which have been capitalized as deferred financing costs in the Company's consolidated balance sheet.
The following unaudited pro forma results for the years ended December 31, 2002 and 2001 are based on the historical financial statements of the Predecessor, adjusted to give effect to the Merger
F-9
and related financing transactions as if the transactions had occurred at the beginning of each period presented:
| |
Year ended December 31 |
|||||
|---|---|---|---|---|---|---|
| |
2002 |
2001 |
||||
| |
(in millions) |
|||||
| Net sales | $ | 1,093.7 | $ | 1,020.1 | ||
| Net income | $ | 33.2 | $ | 7.7 | ||
The Merger was financed through:
In connection with the Merger, Holdings contributed the proceeds from the sale of the Preferred Shares and the sale of the Senior Notes, totaling $200.0 million, to WH Intermediate as capital. Immediately upon the consummation of the Merger, WH Intermediate assumed indirectly through one of its subsidiaries the liability of $7.2 million of expenses relating to the merger and related financing transactions from Holdings, resulting in a net capital contribution of $192.8 million.
2. Basis of Presentation
The Company's financial statements refer to Herbalife and its subsidiaries for periods through July 31, 2002 and to Holdings and its subsidiaries for periods subsequent to July 31, 2002. In addition, "Predecessor" refers to Herbalife and its subsidiaries for periods through July 31, 2002 and "Successor" refers to Holdings and its subsidiaries for periods subsequent to July 31, 2002. The Successor financial statement also includes interest expense and amortization of debt issuance costs incurred prior to the consummation of the Merger.
New Accounting Pronouncements
In December 2003, the SEC issued Staff Accounting Bulletin (SAB) No. 104, "Revenue Recognition," which codifies, revises, and rescinds certain sections of SAB No. 101, "Revenue Recognition," in order to make this interpretive guidance consistent with current authoritative accounting and auditing guidance and SEC rules and regulations. The changes noted in SAB No. 104 did not have a material effect on our consolidated financial statements.
F-10
In May 2003, the FASB issued SFAS 150, "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity." SFAS 150 establishes standards on the classification and measurement of certain instruments with characteristics of both liabilities and equity. SFAS 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. SFAS 150 requires the classification of any financial instruments with a mandatory redemption feature, an obligation to repurchase equity shares, or a conditional obligation based on the issuance of a variable number of its equity shares, as a liability. The adoption of SFAS 150 did not have a material effect on the consolidated financial statements.
In April 2003, the FASB issued SFAS 149, "Amendment of Statement 133 on Derivative Instruments and Hedging Activities," effective for contracts entered into or modified after June 30, 2003. This amendment clarifies when a contract meets the characteristics of a derivative, clarifies when a derivative contains a financing component, and amends certain other existing pronouncements. The adoption of SFAS 149 did not have a material effect on the consolidated financial statements.
In January 2003, the FASB issued Interpretation No. 46 ("FIN 46"), "Consolidation of Variable Interest Entities, an interpretation of ARB No. 51," which addresses consolidation by business enterprises of variable interest entities ("VIEs") either: (1) that do not have sufficient equity investment at risk to permit the entity to finance its activities without additional subordinated financial support, or (2) in which the equity investors lack an essential characteristic of a controlling financial interest. In December 2003, the FASB completed deliberations of proposed modifications to FIN 46 ("Revised Interpretations") resulting in multiple effective dates based on the nature as well as the creation date of the VIE. VIEs created after January 31, 2003, but prior to January 1, 2004, may be accounted for either based on the original interpretation or the Revised Interpretations. VIEs created after January 1, 2004, must be accounted for under the Revised Interpretations. Special Purpose Entities ("SPEs") created prior to February 1, 2003 may be accounted for under the original or revised interpretation's provisions. Non-SPEs created prior to February 1, 2003, should be accounted for under the Revised Interpretation's provisions. The Revised Interpretations are effective for periods after June 15, 2003 for VIEs in which the Company holds a variable interest it acquired before February 1, 2003. For entities acquired or created before February 1, 2003, the Revised Interpretations are effective no later than the end of the first reporting period that ends after March 15, 2004, except for those VIEs that are considered to be special-purpose entities, for which the effective date is no later than the end of the first reporting period that ends after December 31, 2003. The adoption of FIN 46 and the Revised Interpretations has not and is not expected to have an impact on the consolidated financial statements.
In November 2002, the FASB issued Interpretation No. 45 ("FIN 45"), "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others," which addresses the disclosure to be made by a guarantor in its interim and annual financial statements about its obligations under guarantees. The disclosure requirements are effective for interim and annual financial statements ending after December 15, 2002. The Company does not have any material guarantees that require disclosure under FIN 45.
FIN 45 also requires the recognition of a liability by a guarantor at the inception of certain guarantees. FIN 45 requires the guarantor to recognize a liability for the non-contingent component of a guarantee, which is the obligation to stand ready to perform in the event that specified triggering
F-11
events or conditions occur. The initial measurement of this liability is the fair value of the guarantee at inception. The recognition of the liability is required even if it is not probable that payments will be required under the guarantee or if the guarantee was issued with a premium payment or as part of a transaction with multiple elements. The initial recognition and measurement provisions are effective for all guarantees within the scope of FIN 45 issued or modified after December 31, 2002. The Company has adopted the disclosure requirements of FIN 45 and will apply the recognition and measurement provisions for all guarantees entered into or modified after December 31, 2002.
As noted above, the Company has adopted the disclosure requirements of FIN 45 and will apply the recognition and measurement provisions for all guarantees entered into or modified after December 31, 2002. For the year ended December 31, 2003, the Company has not entered into any guarantees within the scope of FIN 45.
Significant Accounting Policies
Consolidation Policy
The consolidated financial statements for the period beginning August 1, 2002 include the accounts of Holdings and its subsidiaries and the periods prior to August 1, 2002 include the accounts of Herbalife and its subsidiaries. All significant intercompany transactions and accounts have been eliminated.
Translation of Foreign Currencies
Foreign subsidiaries' asset and liability accounts are translated for consolidated financial reporting purposes into U.S. dollar amounts at year-end exchange rates. Revenue and expense accounts are translated at the average rates during the year. Foreign exchange translation adjustments are included in accumulated other comprehensive income (loss) on the accompanying consolidated balance sheets. Transaction losses, which include the cost of forward exchange and option contracts, were $0.5 million, $0.4 million, $1.4 million, and $6.5 million for the year ended December 31, 2001, the seven months ended July 31, 2002, the five months ended December 31, 2002, and the year ended December 31, 2003, respectively, and are included in marketing, distribution and administrative expenses in the accompanying consolidated statement of income.
Forward Exchange Contracts and Option Contracts
The Company enters into forward exchange contracts and option contracts in managing its foreign exchange risk on sales to distributors, purchase commitments denominated in foreign currencies, intercompany transactions and bank loans. The Company also enters into interest rate caps in managing its interest rate risk on its variable rate term loan. The Company does not use the contracts for trading purposes.
The Company has adopted SFAS 133, "Accounting for Derivative Instruments and Hedging Activities." SFAS 133, as amended and interpreted, established accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and for hedging activities. All derivatives, whether designated as hedging relationships or not, are required to be recorded on the balance sheet at fair value. If the derivative is designated as a fair-value hedge, the changes in the fair value of the derivative and the underlying hedged item are recognized concurrently
F-12
in earnings. If the derivative is designated as a cash-flow hedge, changes in the fair value of the derivative are recorded in other comprehensive income ("OCI") and are recognized in the statement of operations when the hedged item affects earnings. SFAS 133 defined new requirements for designation and documentation of hedging relationships as well as ongoing effectiveness assessments in order to use hedge accounting. For a derivative that does not qualify as a hedge, changes in fair value are recognized concurrently in earnings.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity of three months or less to be cash equivalents. Cash and cash equivalents are comprised primarily of money market accounts and foreign and domestic bank accounts. To reduce its credit risk, the Company monitors the credit standing of the financial institutions that hold the Company's cash and cash equivalents.
Restricted Cash
The Company's restricted cash pertains to a payment reserve account used to provide payment of scheduled interest and other amounts due on the Senior Notes until March 31, 2005. All amounts deposited are pledged to the Bank of New York as collateral agent for the benefit of the holders of the Senior Notes.
Marketable Securities
The Company's marketable securities are classified as "available for sale." Fluctuations in fair value are included in accumulated other comprehensive loss on the accompanying consolidated balance sheet. Marketable securities at December 31, 2002 are comprised primarily of tax-exempt municipal bonds.
Accounts Receivable
Accounts receivable consist principally of receivables from credit card companies, arising from the sale of product to the Company's distributors, and receivables from importers, who are utilized in a limited number of countries to sell products to distributors. Due to the geographic dispersion of its credit card receivables, the collection risk is not considered to be significant. Although receivables from importers can be significant, the Company performs ongoing credit evaluations of its importers and maintains an allowance for potential credit losses. The Company believes that it provides adequate allowances for receivables from its distributors.
Fair Value of Financial Instruments
The Company has estimated the fair value of its financial instruments using the following methods and assumptions:
F-13
Inventories
Inventories are stated at lower of cost (on the first-in, first-out basis) or market.
Long-Lived Assets
Depreciation of furniture, fixtures, and equipment (including computer hardware and software) is computed on a straight-line basis over the estimated useful lives of the related assets, which range from three to five years. Leasehold improvements are amortized on a straight-line basis over the life of the related asset or the term of the lease, whichever is shorter.
Long-lived assets are reviewed for impairment, based on undiscounted cash flows, whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Measurement of an impairment loss is based on the estimated fair market value of the asset.
Goodwill and intangible assets with indefinite lives are evaluated on an annual basis for impairment, or more frequently if events or changes in circumstances indicate that the asset might be impaired. Intangible assets with finite lives are amortized over their expected lives, which are three years for the distributor network, five years for product formulas and two years for product certifications. The annual amortization expense for intangibles is $0.2 million (2001), $1.5 million (2002), $34.5 million (2003), $23.9 million (2004), $14.0 million (2005), $3.1 million (2006), and $1.8 million (2007).
Income Taxes
Income tax expense includes income taxes payable for the current year and the change in deferred income tax assets and liabilities for the future tax consequences of events that have been recognized in the Company's financial statements or income tax returns. A valuation allowance is recognized to reduce the carrying value of deferred income tax assets if it is believed to be more likely than not that a component of the deferred income tax assets will not be realized.
Royalty Overrides
An independent distributor may earn commissions, called royalty overrides or production bonuses, based on retail volume. Such commissions are based on the retail sales volume of certain other members of the independent sales force who are sponsored by the distributor. In addition, such commissions are recorded when the products are shipped.
F-14
Revenue Recognition
Revenue is recognized when products are shipped and title passes to the Independent Distributor or importer. Product sales are after a discount referred to as "Distributor Allowances." Amounts billed for freight and handling costs are included in net sales. The Company generally receives the net sales price in cash or through credit card payments at the point of sale. Related royalty overrides and allowances for product returns are recorded when the merchandise is shipped. Advanced sales deposits represent prepaid orders for which the Company has not shipped the merchandise.
Accounting for Stock Options
In December 2002, the FASB issued SFAS 148 "Accounting for Stock Based Compensation—Transition and Disclosure." SFAS 148 amends SFAS 123, "Accounting for Stock-Based Compensation," to provide alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. In addition, SFAS 148 amends the disclosure requirements of SFAS 123 to require prominent disclosures in both annual and interim financial statements about the method of accounting for stock-based employee compensation and the effect of the method used on reported results.
The Company applies the intrinsic-value-based method of accounting prescribed by Accounting Principles Board ("APB") Opinion No. 25, "Accounting for Stock Issued to Employees," and related interpretations including FASB Interpretation No. 44, "Accounting for Certain Transactions Involving Stock Compensation," an interpretation of APB Opinion No. 25, issued March 2000, to account for its stock-based awards for employees. For options granted to employees, compensation expense is recorded on the date of grant only if the current market price of the underlying stock exceeded the exercise price. SFAS 123 established accounting and disclosure requirements using a fair-value-based method of accounting for stock-based employee compensation plans. As allowed by SFAS 123, the Company has elected to continue to apply the intrinsic-value-based method of accounting described above, and has adopted only the disclosure requirements of SFAS 123.
The following tables illustrate the effect on net income if the fair-value-based method had been applied to all outstanding and unvested awards in each period:
| |
2001 |
2002 |
2003 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, |
January 1 to July 31 |
August 1 to December 31 |
Year ended December 31, |
|||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
|||||||||
| |
(dollars in millions) |
||||||||||||
| Net income as reported | $ | 42.6 | $ | 9.2 | $ | 14.0 | $ | 36.8 | |||||
| Add: Stock-based employee compensation expense included in reported net income | — | 0.6 | — | 1.1 | |||||||||
| Deduct: Stock-based employee compensation expense determined under fair value based methods for all awards | (1.2 | ) | (0.4 | ) | — | (0.7 | ) | ||||||
| Pro forma net income | $ | 41.4 | $ | 9.4 | $ | 14.0 | $ | 37.2 | |||||
F-15
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions. Such estimates and assumptions affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Reclassifications
Certain reclassifications were made to the prior year financial statements to conform to the current year presentation.
3. Inventories
Inventories consist primarily of finished goods available for resale and can be categorized as follows:
| |
December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
|||||
| |
(dollars in millions) |
||||||
| Weight management and inner nutrition | $ | 41.5 | $ | 44.2 | |||
| Outer Nutrition® | 8.2 | 7.0 | |||||
| Literature, promotional and others | 7.2 | 8.2 | |||||
| Total | $ | 56.9 | $ | 59.4 | |||
4. Long-Term Debt
Long-term debt consists of the following:
| |
At December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
|||||
| |
(dollars in millions) |
||||||
| Senior subordinated notes | $ | 163.0 | $ | 158.2 | |||
| Borrowing under senior credit facility | 135.0 | 119.8 | |||||
| Senior Notes | 38.5 | 39.6 | |||||
| Discount—Senior note warrant | (1.7 | ) | (1.6 | ) | |||
| Capitalized leases | 3.3 | 5.5 | |||||
| Other debt | 2.7 | 3.8 | |||||
| 340.8 | 325.3 | ||||||
| Less: current portion | 19.2 | 72.4 | |||||
| $ | 321.6 | $ | 252.9 | ||||
F-16
Interest expense was $0.4 million, $0.2 million, $25.2 million, and $41.2 million for the year ended December 31, 2001, the seven months ended July 31, 2002, the five months ended December 31, 2002, and the year ended December 31, 2003.
In connection with the Merger, the Company consummated certain related financing transactions, including the issuance by WH Acquisition on June 27, 2002 of $165.0 million of 113/4% Senior Subordinated Notes (the "Senior Subordinated Notes") issued at 98.716% of par, due July 15, 2010. Interest on the Senior Subordinated Notes is to be paid semi annually on January 15th and July 15th, of each year (the first payment of which was made on January 15, 2003). In connection with this financing, the Company incurred $25.1 million of debt issuance costs, which are being amortized, over the term of the debt using the effective interest rate method. During the third quarter of 2003, the Company repurchased $5 million principal value of Senior Subordinated Notes. The fair value of the Senior Subordinated Notes was $189.6 million and $165.0 million at December 31, 2003 and 2002, respectively.
In addition, the Company, as borrower, entered into a Credit Agreement dated as of July 31, 2002 with the guarantors party, the lenders party, Rabobank International, as documentation agent, General Electric Capital Corporation, as syndication agent, UBS Securities LLC (successor in interest to UBS Warburg LLC), as arranger, and UBS AG, Stamford Branch, as administrative agent, collateral agent and issuing bank (the "Credit Agreement"), which provides for a term loan amount of $180.0 million and a revolving credit facility in the amount of $25.0 million (collectively, the "Senior Credit Facility"). In conjunction with this financing, the Company incurred $16.7 million of debt issuance costs, which are being amortized over the term of the debt using the effective interest method. The revolving credit facility is available until July 31, 2007. The term loan and the revolving credit facility bear interest, at the option of the Company, at either the alternate base rate or the LIBOR rate plus in each case an applicable margin. The base rate applicable margin for the term loan is 3.00%, while the LIBOR rate applicable margin is 4.00%. As of December 31, 2003, no amounts had been borrowed under the revolving credit facility. As of December 31, 2003, the Company had selected the LIBOR rate alternative (three months) with the December 31, 2003 interest rate of 5.1%. The base rate applicable margin for the revolving credit facility is 2.75%, while the LIBOR rate applicable margin is 3.75%. In accordance with the terms of the Senior Credit Facility, on October 30, 2002, Herbalife purchased a three-year 5% LIBOR interest rate cap covering $34.4 million of the term loan.
Also, in connection with the Merger, the Company issued and sold $38.0 million principal amount of 15.5% Senior Notes ("Senior Notes"), due July 15, 2011. The Senior Notes accrue interest at the rate of 15.5% per annum. Interest is required to be paid on March 31, June 30, September 30, and December 31 in each year commencing September 30, 2002. In accordance with the terms of the Senior Notes, 12.5% per annum of the interest payable quarterly is to be paid in cash and 3.0% per annum of the interest payable quarterly is to be paid through the issuance of additional notes. The principal amount of the Senior Notes is required to be paid on July 15, 2011. From the net proceeds of the issuance of the Senior Notes, the Company established and deposited $12.5 million to a payment reserve account to provide payment when due of scheduled cash interest payments until March 31, 2005 and, in certain circumstances, other amounts due on the Senior Notes. All amounts deposited in the payment reserve account are pledged by the Company to The Bank of New York, as collateral agent for the benefit of the holders of the Senior Notes. The balance of the payment reserve account was $5.7 million at December 31, 2003, and it was reflected in the restricted cash balance on the balance sheet.
F-17
In connection with the sale of the Company's Senior Notes, the Company issued warrants for approximately 2.0 million preferred shares to the purchasers of the Senior Notes. Affiliates of Whitney hold warrants with the right to purchase 0.9 million preferred shares. The warrants may be exercised at any time at a price of $0.01 per share. The warrants also have customary protection against dilution. The Company allocated $1.8 million as capital surplus for the issuance of the warrants on July 31, 2002. The Senior Notes were discounted by the same amount and such discount will be amortized over the term of the Senior Notes.
The Senior Subordinated Notes and the Senior Credit Facility are guaranteed by the Guarantors (as defined in Note 15 herein). The Senior Credit Facility is also guaranteed by Holdings. The obligations under the Senior Credit Facility are secured by (i) first priority pledges of (A) all of the shares of the Guarantors and (B) 65% of the equity interests of the foreign subsidiaries of Herbalife that are not Guarantors other than HIIP Investment Co., LLC, Herbalife Foreign Sales Corporation, Importadora Y Distribuidora Herbalife International de Chile Limitada, Herbalife International Greece S.A., Herbalife Hungary Trading, Limited, PT Herbalife Indonesia, Herbalife International SBN, BHD, HBL International Maroc S.à.R.L., Herbalife International Products N.V., Herbalife International Holdings, Inc., Herbalife International, S.A., Herbalife Dominicana, S.A., and Herbalife Del Ecuador, S.A. and (ii) security interests in and liens on all accounts receivable, inventory and other property and assets of Holdings and the Guarantors (other than the escrow account for interest on the Senior Notes).
The Senior Subordinated Notes, the Senior Credit Facility and the Senior Notes include customary covenants that restrict, among other things, the ability to incur additional debt, pay dividends or make certain other restricted payments, incur liens, merge or sell all or substantially all of the assets, or enter into various transactions with affiliates. Additionally, the Senior Credit Facility includes covenants relating to the maintenance of certain leverage, fixed charge coverage, and interest coverage ratios.
In December 2002, the Company and its lenders amended the Credit Agreement. Under the terms of the amendment, the Company made a prepayment of $40.0 million on December 30, 2002. In connection with this prepayment, the lenders waived the December 31, 2002 and March 31, 2003 mandatory amortization payments of $7.5 million along with a mandatory 50% excess cash flow payment solely for the year ended December 31, 2002. The Company's debt agreement has a provision that requires the Company to make early payments to the extent of excess cash flow, as defined. In addition, Herbalife was allowed to pay certain monitoring fees to Whitney and Golden Gate that under the terms of the original Credit Agreement were to be accrued, but not paid, until the obligations under the Credit Agreement had been paid in full. The amortization of the principal payments was also modified so that Herbalife was obligated to pay approximately $2.2 million on June 30, 2003 and will pay $6.5 million in each subsequent quarter through March 31, 2008, with the final payment on June 30, 2008 being approximately $8.7 million. As of December 31, 2003, Herbalife anticipated a $40 million mandatory excess cash flow payment in the first quarter of 2004. Consequently, the future quarterly principal payments will be reduced. However, the Company may be obligated to make future excess cash flow payments as described above. In addition, Herbalife was granted the right to make prepayments, of up to $25 million in aggregate principal amount, on the Senior Subordinated Notes over the life of the Credit Agreement provided that Herbalife meets an agreed upon leverage ratio.
F-18
As of December 31, 2003, certain Whitney affiliated companies had provided funding for $5.1 million of the term loan under the Senior Credit Facility, $1.3 million of the Senior Subordinated Notes and held $17.3 million of the Senior Notes.
Annual scheduled payments of long-term debt are: $72.4 million (2004), $17.5 million (2005), $15.5 million (2006), $15.0 million (2007), $8.7 (2008), and $196.2 million (thereafter).
5. Lease obligations
The Company has warehouse, office, furniture, fixtures and equipment leases, which expire at various dates through 2011. Under the lease agreements, the Company is also obligated to pay property taxes, insurance, and maintenance costs.
Certain of the leases contain renewal options. Future minimum rental commitments for non-cancelable operating leases and capital leases at December 31, 2003 were as follows:
| |
Operating |
Capital |
||||
|---|---|---|---|---|---|---|
| |
(in millions) |
|||||
| 2004 | $ | 12.6 | $ | 3.3 | ||
| 2005 | 9.4 | 1.9 | ||||
| 2006 | 4.7 | 0.5 | ||||
| 2007 | 1.4 | — | ||||
| 2008 | 1.2 | — | ||||
| Thereafter | 0.4 | — | ||||
| Total | $ | 29.7 | 5.7 | |||
| Less: amounts included above representing interest | 0.2 | |||||
| Present value of net minimum lease payments | $ | 5.5 | ||||
Rental expense for 2001, the seven months ended July 31, 2002, the five month period ended December 31, 2002 and the year ended December 31, 2003 was $20.0 million, $11.6 million, $8.6 million, and $21.0 million, respectively.
Property under capital leases is included in property on the accompanying consolidated balance sheets as follows: (in millions)
| |
December 31 |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
||||||
| |
(in millions) |
|||||||
| Equipment | $ | 9.9 | $ | 10.2 | ||||
| Less: accumulated depreciation | (6.8 | ) | (4.7 | ) | ||||
| Total | $ | 3.1 | $ | 5.5 | ||||
6. Employee compensation plans
The Company maintains a profit sharing plan pursuant to Sections 401 (a) and (k) of the Internal Revenue Code. The plan is available to substantially all employees who meet length of service
F-19
requirements. Employees may elect to contribute 2% to 17% of their compensation, and the Company will match 3% of the earnings of each employee who elects to defer 2% or more of his or her earnings. Participants are partially vested in the Company contributions after one year and fully vested after five years. The Company contributed $1.3 million, $0.8 million, $0.6 million, and $1.3 million for the year ended December 31, 2001, the seven months ended July 31, 2002, the five months ended December 31, 2002, and the year ended December 31, 2003, respectively.
The Company has two non-qualified, deferred compensation plans for select groups of management: the "Management Plan" and the "Senior Executive Plan." The deferred compensation plans allow eligible employees to elect annually to defer up to 50% of their base annual salary and up to 100% of their annual bonus for each calendar year (the "Annual Deferral Amount"). The Company makes matching contributions on behalf of each participant in the Senior Executive Plan. Effective for 2002, the Senior Executive Plan was amended to provide that the amount of the matching contributions is to be determined by the Company in its discretion. For 2003, the matching contribution was 3% of a participant's base salary.
Each participant in either of the two deferred compensation plans has at all times a fully vested and non-forfeitable interest in each year's contribution, including interest credited thereto, and in any Company matching contributions, if applicable. In connection with a participant's election to defer an Annual Deferral Amount, the participant may also elect to receive a short-term payout, equal to the Annual Deferral Amount plus interest. Such amount is payable in two or more years from the first day of the year in which the Annual Deferral Amount is actually deferred.
In July 2002, the Company adopted an additional deferred compensation plan, the ("Supplemental Plan"). The Supplemental Plan allows employees to participate, who are highly compensated and who are eligible to participate in the Change in Control Plan. The administrative committee that manages and administers the plans (the "Deferred Compensation Committee") allows eligible employees to defer up to 100% of their Change in Control Payments.
Each participant in the Supplemental Plan will be deemed to have invested in funds that provide a return equal to the short-term AFR, within the meaning of the code. The entire interest of each participant in the Supplemental Plan is always fully vested and non-forfeitable. In connection with a participant's election to defer the Change in Control Payment, the participant may also elect to receive a short-term payout, equal to the deferral amount plus earnings, which is payable two or more years from the first day of the year in which the deferral amount is actually deferred. Subject to the short-term payout provision and specified exceptions for unforeseeable financial emergencies, a participant may not withdraw, without incurring a ten percent (10%) withdrawal penalty, all or any portion of his or her account under the Supplemental Plan prior to the date that such participant either (1) is determined by the Deferred Compensation Committee to have incurred permanent and total disability of (2) dies or otherwise terminates employment with the Company.
The total deferred compensation expense of the three deferred compensation plans net of participant contributions was $3.9 million, $1.9 million, $1.3 million, and $1.0 million for 2001, seven months ended July 31, 2002, five months ended December 31, 2002, and the year ended December 31, 2003, respectively. The total long-term deferred compensation liability under the three deferred compensation plans was $22.4 million and $26.2 million at December 31, 2003 and 2002.
F-20
The Company has an Executive Retention Plan. The purpose of the Executive Retention Plan is to provide financial incentives for a select group of management and highly compensated employees of the Company to continue to provide services to the Company during the period immediately before and immediately after change in control, as defined.
As a result of certain actions by Herbalife's Board, the Merger was not deemed to be a Change in Control under the Executive Retention Plan. Thus, the consummation of the Merger did not result in the payment of any benefit pursuant to the Executive Retention Plan.
The Company also established an Executive Retention Trust to provide benefits under the Executive Retention Plan. The Executive Retention Trust is an irrevocable trust established with an institutional trustee. The Administrative Committee of the Executive Retention Plan will establish an individual account in the Executive Retention Trust for each participant in the Executive Retention Plan. Until the occurrence of a change in control, the Administrative Committee will control the investment of the assets in the Executive Retention Trust, and will determine the allocation of the assets of the Executive Retention Trust to the individual accounts of participants. Each participant who qualifies for a benefit under the Executive Retention Plan will receive a lump sum benefit equal to the dollar amount in his or her individual account in the Executive Retention Trust. The benefit shall be paid within 90 days after the participant qualifies for the benefit. If a participant's employment with the Company terminates before the participant qualifies for a benefit under the Executive Retention Plan, the participant's account in the Executive Retention Trust will revert to the Company. A participant's benefit under the Executive Retention Plan will be reduced if the amount would cause payment of federal excise tax.
The deferred compensation plans are unfunded and their benefits are paid from the general assets of the Company, except that the Company has contributed to a "rabbi trust" whose assets will be used to pay the benefits if the Company remains solvent, but can be reached by the Company's creditors if the Company becomes insolvent. The value of the assets in the "rabbi trust" was $18.5 million and $27.5 million as of December 31, 2003 and 2002, respectively. The Company has also contributed to the Executive Retention Trust, which is an irrevocable trust. This irrevocable trust's assets will be used to pay the benefits of the Executive Retention Plan and are not intended to be reachable by the Company's creditors. The value of the assets in the irrevocable trust was $2.8 million and $4.5 million as of December 31, 2003 and 2002, respectively.
The Company had two Change in Control Plans for Senior Management, a Change in Control Plan and a Management Employee Change in Control Plan. Pursuant to the agreements in place prior to the signing of the Merger Agreement, upon consummation of the Merger, certain of the Company's executives received change in control payments (after making necessary adjustments for purposes of Section 280G and 4999 of the Code) of $7.6 million. Pursuant to the Herbalife Management Employee Change in Control Plan, which was in place prior to signing of the Merger Agreement, eligible employees that within one year after the occurrence of a Change in Control were involuntarily terminated by the Company would be entitled to receive one year of their base compensation. The agreement expired on July 31, 2003. As a result of the Merger, the Change in Control Payments were made and expensed in July 2002.
F-21
7. Retirement plan
The Company had a nonqualified, non-contributory Supplemental Executive Retirement Plan ("SERP") providing retirement benefits for a select group of management and highly compensated employees. The normal retirement benefit under the SERP is 60 quarterly installment payments commencing at age 65, each of which equals one-quarter of 2% of "compensation" times the number of years of participation up to 20 years. A participant becomes fully vested in his or her interest in the SERP on his or her normal or early retirement date, death, or disability, or on a change in control of the Company. If a participant's employment is terminated for cause, the Company has the discretion to reduce his or her vested benefit to zero. In all other cases, a participant's vested interest is zero until he or she has completed five years of participation, and gradually increases to 100% when he or she has completed nine years of participation. The Plan Administrator has the discretion to credit a participant with additional years of participation as of his or her date of hire or commencement of participation in the SERP. The SERP was completely curtailed effective December 31, 2002. At December 31, 2002 the liability to SERP participants was $5.9 million and participants either received a cash payment in the first quarter of 2003 or a rollover to the Company's deferred compensation plan on January 1, 2003. The following table shows the net periodic pension cost and other data about the SERP:
| |
2002 |
||||
|---|---|---|---|---|---|
| |
(in millions) |
||||
| Change in benefit obligation | |||||
| Benefit obligation at beginning of year | $ | 11.4 | |||
| Service cost | 1.4 | ||||
| Interest cost | 0.7 | ||||
| Amendments | (3.1 | ) | |||
| Actuarial (gain) loss | (3.1 | ) | |||
| Benefits paid | (1.4 | ) | |||
| Benefit obligation at end of year | $ | 5.9 | |||
| Funded status | $ | (5.9 | ) | ||
| Unrecognized actuarial loss | — | ||||
| Unrecognized prior service cost | — | ||||
| Net amount recognized | $ | (5.9 | ) | ||
| Amounts recognized in the consolidated balance sheets | |||||
| Consists of: | |||||
| Accrued benefit liability | $ | (5.9 | ) | ||
| Intangible asset | — | ||||
| Net amount recognized | $ | (5.9 | ) | ||
Weighted-average assumptions as of December 31 |
|||||
| Discount rate | N/A | ||||
| Rate of compensation increase | N/A | ||||
F-22
| |
2001 |
2002 |
||||
|---|---|---|---|---|---|---|
Components of net periodic benefit cost |
||||||
| Service cost | $ | 1.4 | $ | 1.4 | ||
| Interest cost | 0.7 | 0.7 | ||||
| Amortization of prior service cost | 0.5 | 0.5 | ||||
| Net periodic pension cost | 2.6 | $ | 2.6 | |||
8. Transactions with related parties
The Company has entered into agreements with Whitney and Golden Gate to pay monitoring fees for their services and other fees and expenses. Under the monitoring fee agreements, the Company is obligated to pay an annual amount of up to $5.0 million, but not less than $2.5 million for an initial period of ten years subject to the provisions in the Credit Agreement as amended. For the period August 1 to December 31, 2002 and for the year ended December 31, 2003, the Company expensed monitoring fees in the amount of $2.1 million and $5.0 million and other expenses of $0.1 million and $3.4 million, respectively. In addition, in connection with the Merger, the Company paid Whitney and Golden Gate $24.1 million in fees, which have been classified in the balance sheet as deferred financing costs and are being amortized.
Selected members of the Company's distributor organization and senior management have purchased, either from Holdings or from the Equity Sponsors, 12% Series A Cumulative Convertible Preferred Shares of Holdings. The price paid by participating members of the Company's distributor organization and senior management to the Equity Sponsors in the August and October 31, 2002 offering was $1.76 per share. The January 31, 2003 offering to members of the Company's President's Team by the Equity Sponsors was $1.97 per share. In connection with the May 20, 2003 offering by the Equity Club, the price paid by members of the Company's President's Team to the Equity Sponsors and by members of the Company's Chairman's Club to Holdings was $2.21 per share. Michael O. Johnson, the Company's Chief Executive Officer, purchased from Holdings 203,620 shares on June 24, 2003. The price paid by Mr. Johnson was the same price paid by members of the Company's distributor organization in the May 20, 2003 offering.
Francis Tirelli, the Company's former Chief Executive Officer, entered into a separation and general release agreement with the Company, effective on December 24, 2002. The preferred shares previously owned by Mr. Tirelli were purchased by certain existing shareholders and in connection therewith, an advance of $0.5 million was made by the Company's subsidiary, Herbalife International of America, Inc., to those shareholders. As of December 31, 2003 $0.3 million is outstanding.
In June 2003, Holdings entered into a subscription agreement with its Chief Executive Officer, Michael Johnson, pursuant to which Holdings has issued 0.2 million newly issued preferred shares at a price of $2.21 per share. The price paid by Mr. Johnson was the same price paid by members of the Company's distributors in a May 2003 offering. In addition, Mr. Johnson was granted options to purchase 5.9 million common shares in five tranches consisting of approximately 1.2 million shares each. The purchase price per share for each separate tranche is $0.44, $1.76, $5.28, $8.80, and $12.32, respectively. The Company has certain repurchase rights with respect to shares acquired upon exercise of these options, as further detailed in Note 10, except that the repurchase rights with respect to shares
F-23
which can be repurchased at the lower of the exercise price or the fair value of the shares lapse on the earlier of the fifth anniversary of the date of grant or on initial public offering of the Company.
9. Contingencies
The Company is from time to time engaged in routine litigation. The Company regularly reviews all pending litigation matters in which it is involved and establishes reserves deemed appropriate by management for these litigation matters when a probable loss estimate can be made.
Herbalife is a defendant in a purported class action lawsuit pending in the U.S. District Court of California (Jacobs v. Herbalife International, Inc., et. Al) originally filed on February 19, 2002 challenging marketing practices of several distributors and Herbalife under various state and federal laws. As a result of recent rulings by the Court, only claims filed under federal securities law remain. The Company understands that the plaintiffs have refilled certain state law claims in the Superior Court of the State of California, County of San Francisco. The Company has not been served with a complaint. The parties are in discussions regarding a possible settlement but no binding settlement agreement has yet been reached. We believe we have meritorious defenses to the suit.
Herbalife and certain of its distributors have been named as defendants in a purported class action lawsuit filed July 16, 2003 in the Circuit Court of Ohio County in the State of West Virginia (Mey v. Herbalife International, Inc., et al). Herbalife removed the lawsuit to federal court and the plaintiff sought to remand the lawsuit to state court. The plaintiff's motion was denied. The complaint alleges that certain telemarketing practices of certain Herbalife distributors violate the Telephone Consumer Protection Act and seek to hold Herbalife liable for the practices of its distributors. The Company believes that it has meritorious defenses to the suit.
As a marketer of dietary and nutritional supplements and other products that are ingested by consumers or applied to their bodies, the Company has been subjected to various product liability claims. The effects of these claims to date have not been material to the Company, and the reasonably possible range of exposure on currently existing claims is not material. The Company believes that it has meritorious defenses to the allegations contained in the lawsuits. The Company currently maintains product liability insurance with an annual deductible of $10.0 million.
Certain of the Company's subsidiaries have been subject to tax audits by governmental authorities in their respective countries. In certain of these tax audits, governmental authorities are proposing that significant amounts of additional taxes and related interest and penalties are due. The Company and its tax advisors believe that there are substantial defenses to their allegations that additional taxes are owing, and the Company is vigorously contesting the additional proposed taxes and related charges. These matters may take several years to resolve, and the Company cannot be sure of their ultimate resolution. However, it is the opinion of management that adverse outcomes, if any, will not likely result in a material effect on our financial condition and operating results.
10. Shareholders' equity
The Company had authorized 103 million preferred shares at $0.001 par value. The Company increased the number of authorized preferred shares from 103 million to 106 million on July 31, 2003. On July 31, 2002, 100 million preferred shares were issued for $176 million in a private placement offering. On May 30, 2003, an additional 1.8 million preferred shares were sold for $4 million in a
F-24
private placement offering. On August 27, an additional 0.2 million preferred shares were sold to the Company's Chief Executive Officer, Michael Johnson, pursuant to a subscription agreement entered into in June, 2003. The preferred shares have dividend provisions and liquidation preference over the common shares. The preferred shares also have redemption rights.
Preferred shares are entitled to receive cash dividends, at a rate per annum equal to 12% of the original issue price. Unpaid dividends will compound on a quarterly basis. All dividends are cumulative from the accrual date, whether or not earned or declared. Upon automatic redemption of the preferred shares, accrued and unpaid dividends shall be paid by the Company, at the election of the Company, in cash or in common shares. Payment of dividends is subject to restrictions imposed by the debt documents. The total undeclared and unpaid accumulative dividend on preferred shares was $32.8 million and $9.0 million on December 31, 2003 and 2002, respectively.
Upon any liquidation, dissolution or winding-up of the Company, each holder of preferred shares will have the right to receive for each preferred share (i) before any distribution or payment to the holder of common shares, the amount equal to the original issue price, plus any accrued and unpaid dividends thereon plus (ii) the amount the holder would have received with respect to such holder's common shares assuming that the preferred shares had been converted into common shares immediately prior to such winding up.
Preferred shares shall automatically convert on the earlier of (x) an initial public offering of the Company and (y) a sale, merger or other change of control event into a unit consisting of (i) the right to receive cash equal to the original issue price per preferred share and (ii) one share of common per preferred share subject to some adjustments.
The terms of the preferred shares contain anti-dilution adjustments for structural events such as stock splits, dividends, combinations, subdivisions, and reclassifications.
The Shareholders' Agreement (to which all shareholders are party) gives the Company and the equity sponsors the right to repurchase shares from employees and distributors of the Company in certain circumstances which include the dismissal, death or retirement of an employee or distributor. The price at which preferred shares may be repurchased is the greater of formula price or cost for a termination without cause, or the lesser of formula price or cost for a termination for cause. The price at which common shares may be repurchased is the greater of current market price or cost for a termination without cause, or the lesser of current market price of cost for a termination for cause.
The Company has entered into arrangements with its stockholders and the holders of its Senior Notes that grant such holders pre-emptive rights, protection from dilution, and certain negative covenants. These arrangements may restrict the ability of the Company to issue additional equity.
F-25
WH Holdings (Cayman Islands) Ltd. Stock Option Plan ("Management Plan"). Holdings has established a stock option plan that provides for the grant of options to purchase common shares of WH Holdings (Cayman Islands) Ltd. to members of management of Herbalife. The option plan is administered by a committee appointed by the Board of Holdings. Upon conversion of the options into common shares of Holdings, members of management of Herbalife will be required to enter into a shareholders' agreement and a registration rights agreement with Holdings.
WH Holdings (Cayman Islands) Ltd. Independent Directors Stock Option Plan ("Independent Directors Plan"). Holdings has established an independent directors stock option plan that provides for the grant of options to purchase common shares of Holdings to independent directors of Holdings. One million shares have been reserved for grant under this plan.
Approximately 15.5% of the share capital at the time of the Merger or 18.7 million shares of Holdings' are available for grants under the two plans. As of December 31, 2003, the Company had granted approximately 17.7 million stock options, of which 0.8 million were under the Independent Directors Plan.
The Management Plan and the Independent Directors Plan (collectively, the "Plans") call for options to be granted with exercise prices not less than the fair value of the underlying shares on the date of grant. Options under the Plans vest and become exercisable in quarterly 5% increments unless provided otherwise by the applicable grant agreement. Options granted under the plans have a contractual life of 10 years and shares issued on exercise are subject to certain repurchase provisions following a termination in employment or directorship.
On November 6, 2003, the Board of the Company approved an amendment to its stock option plan under the WH Holdings (Cayman Islands) Ltd. Stock Incentive Plan with certain senior management employees ("Senior Plan"). Under the previous terms, the Company determined that the options did not substantively vest since they could be repurchased by the Company at the lower of fair market value or exercise price. Accordingly, the Company concluded that there would not be a measurement date until a liquidity event occurred when the Company's repurchase right would expire for GAAP purposes under the plan and no compensation expense was recognized. The Company has also concluded that the amendments result in a fixed plan with a measurement date as of November 6, 2003. Based on the estimated fair value of the Company's common shares, the Company believes the fair value of the common shares issuable upon exercise of the options exceeded the exercise price per share for the options on this date and recorded a compensation charge to account for the indicated intrinsic value. The total intrinsic value and the related compensation expense is $9.4 million which will be recognized over a 7-year period following the date of grant, beginning with $1.2 million in the fourth quarter of 2003, representing the portion of the options that have already vested, and $1.3 million per year until fully expensed.
Under the Management Plan, upon termination of employment, excluding senior executives, Holdings and the institutional shareholders will have the right to repurchase the shares acquired upon the exercise of options within a specified period at a price not less than (a) the fair market value at the time of termination or (b) the exercise price, however, the right to repurchase at the exercise price shall lapse at the rate of 20% per annum. Upon termination of a senior executive, Holdings and the institutional shareholders have the right to repurchase the shares if such termination (i) was voluntary, due to resignation or for cause (A) before the seventh anniversary of the option grant, at an amount
F-26
equal to the lesser of: (a) the fair market value at the time of repurchase or (b) after the seventh anniversary of the option grant, at an amount equal to the fair market value at the time of repurchasing; or (ii) was involuntary without cause or because of death, retirement or disability at an amount equal to the greater of: (a) the fair market value at the time of such termination; or (b) the exercise price.
Under the Independent Directors Plan, upon termination of an Independent Director, Holdings and the institutional shareholders have the right to repurchase the shares if such termination (i) was voluntary, due to resignation or for cause at an amount equal to the lesser of: (a) the fair market value at the time of such termination; or (b) the exercise price; (ii) was involuntary without cause or because of death, retirement or disability at an amount equal to the greater of: (a) the fair market value at the time of such termination; or (b) the exercise price.
Option groups outstanding at December 31, 2001, July 31, 2002, December 31, 2002, December 31, 2003 and related option information is as follows:
| |
WH Holdings common shares |
||||||
|---|---|---|---|---|---|---|---|
| 2003 (successor) |
Options |
Weighted average exercise price |
|||||
| |
(in millions) |
|
|||||
| Outstanding at January 1, 2003 | 6.7 | $ | 1.18 | ||||
| Granted | 12.1 | 4.08 | |||||
| Exercised | — | — | |||||
| Cancelled | (1.1 | ) | 1.15 | ||||
| Outstanding at December 31 | 17.7 | $ | 3.17 | ||||
| Available for grant at December 31 | 1.0 | ||||||
| Total reserved shares | 18.7 | ||||||
| Exercisable at December 31 | 2.5 | $ | 1.14 | ||||
| Option prices per share | |||||||
| Granted | $0.44 - | $12.32 | |||||
| Exercised | — | ||||||
| Weighted average fair value of options granted during the year | $0.24 | ||||||
F-27
| |
WH Holdings common shares |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2002 (successor) |
Options |
Weighted average exercise price |
||||||
| |
(in millions) |
|
||||||
| Granted | 6.7 | $ | 1.18 | |||||
| Exercised | — | — | ||||||
| Canceled | — | — | ||||||
| Outstanding at December 31 | 6.7 | $ | 1.18 | |||||
| Available for grant at December 31 | 12.0 | |||||||
| Total reserved shares | 18.7 | |||||||
| Exercisable at December 31 | 0.3 | $ | 1.19 | |||||
| Option prices per share | ||||||||
| Granted | $0.44 - | $1.76 | ||||||
| Exercised | — | |||||||
| Weighted average fair value of options granted during the year | $0.03 | |||||||
| |
Class A stock |
Class B stock |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2002 (predecessor) |
Options |
Weighted average exercise price |
Options |
Weighted average exercise price |
|||||||||
| |
(in millions) |
|
|
|
|||||||||
| |
|
|
|
(in millions) |
|
||||||||
| Outstanding at January 1 | 0.9 | $ | 7.88 | 3.8 | $ | 7.33 | |||||||
| Granted | — | — | — | — | |||||||||
| Exercised | (0.3 | ) | 7.90 | (1.1 | ) | 6.85 | |||||||
| Canceled | — | — | — | — | |||||||||
| Converted | (0.6 | ) | 7.86 | (2.7 | ) | 7.54 | |||||||
| Outstanding at July 31 | — | $ | — | — | $ | — | |||||||
| Available for grant at July 31 | — | — | |||||||||||
| Total reserved shares | — | — | |||||||||||
| Exercisable at July 31 | — | $ | — | — | $ | — | |||||||
| Option prices per share | |||||||||||||
| Granted | — | — | |||||||||||
| Exercised | $7.38 - | $8.00 | $ | 6.63 | |||||||||
| Weighted average fair value of options granted during the year | — | — | |||||||||||
F-28
| |
Class A stock |
Class B stock |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2001 (predecessor) |
Options |
Weighted average exercise price |
Options |
Weighted average exercise price |
|||||||||||
| |
(in millions) |
|
|
|
|
||||||||||
| |
|
|
|
(in millions) |
|
||||||||||
| Outstanding at January 1 | 2.0 | $ | 7.89 | 4.6 | $ | 6.67 | |||||||||
| Granted | — | — | 0.5 | 11.30 | |||||||||||
| Exercised | (1.1 | ) | 7.89 | (1.3 | ) | 6.52 | |||||||||
| Canceled | — | — | — | — | |||||||||||
| Outstanding at December 31 | 0.9 | $ | 7.88 | 3.8 | $ | 7.33 | |||||||||
| Available for grant at December 31 | 0.1 | 0.2 | |||||||||||||
| Total reserved shares | 1.0 | 4.0 | |||||||||||||
| Exercisable at December 31 | 0.9 | $ | 7.87 | 2.7 | $ | 6.75 | |||||||||
| Option prices per share | |||||||||||||||
| Granted | — | $ | 11.30 | ||||||||||||
| Exercised | $7.38 - | $8.00 | $ | 6.63 - | $ | 8.63 | |||||||||
| Weighted average fair value of options granted during the year | — | $ | 3.14 | ||||||||||||
The fair value of the stock options granted during the periods presented was determined using the Black-Scholes option pricing model and the following weighted average assumptions:
| |
2001 |
2002 |
2003 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31 |
January 1 to July 31 |
August 1 to December 31 |
Year ended December 31 |
||||||||
| |
(Predecessor) |
(Predecessor) |
(Successor) |
(Successor) |
||||||||
| |
Class A |
Class B |
Class A |
Class B |
Common |
Common |
||||||
| Risk free interest rate | N/A | 2.92% | N/A | N/A | 3.20% | 3.00% | ||||||
| Expected option life | N/A | 3.0 years | N/A | N/A | 5.0 years | 5.0 years | ||||||
| Volatility | N/A | 56.67% | N/A | N/A | 0.00% | 0.00% | ||||||
| Dividend yield | N/A | 6.50% | N/A | N/A | 0.00% | 0.00% | ||||||
The following table summarizes information regarding option groups outstanding at December 31, 2003:
| Range of Exercise Price |
Options outstanding |
Weighted average remaining contractual life |
Weighted average exercise price |
Options exercisable |
Weighted average exercise price |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
|
|
(in millions) |
|
|||||||
| $0.44 | 6.1 | 9.1 | $ | 0.44 | 1.3 | $ | 0.44 | |||||
| $1.76 | 5.7 | 9.0 | $ | 1.76 | 1.1 | $ | 1.76 | |||||
| $2.50 - $11.50 | 4.6 | 9.4 | $ | 6.03 | 0.1 | $ | 2.82 | |||||
| $12.32 | 1.3 | 9.3 | $ | 12.32 | — | — | ||||||
F-29
11. Segment Information
The Company is a network marketing company that sells a wide range of weight management products, nutritional supplements and personal care products within one industry segment as defined under SFAS 131, "Disclosures about Segments of an Enterprise and Related Information." The Company's products are manufactured by third party providers and then sold to independent distributors who sell Herbalife products to retail consumers or other distributors.
The Company has operations throughout the world and is organized and managed by geographic area. In the first quarter of 2003, the Company elected to aggregate its operating segments into one reporting segment, except U.S. and Japan, as management believes that the operating segments have similar operating characteristics and similar long term operating performance. In making this determination, management believes that the operating segments are similar in the nature of the products sold, the product acquisition process, the types of customers sold to, the methods used to distribute the products, and the nature of the regulatory environment. However, the Company does recognize revenue from sales to distributors in four geographic regions: The Americas, Europe, Asia/Pacific Rim, and Japan.
"Net sales" represents product sales including handling and freight income. The Company receives its net sales price in cash or through credit card payments upon receipt of orders from distributors.
F-30
| |
2001 |
2002 |
2003 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31 |
January 1 to July 31 |
August 1 to December 31 |
Year ended December 31 |
||||||||||
| |
(Predecessor) |
(Predecessor) |
(Successor) |
(Successor) |
||||||||||
| |
(in millions) |
|||||||||||||
| Net Sales: | ||||||||||||||
| United States | $ | 278.8 | $ | 189.1 | $ | 117.3 | $ | 274.9 | ||||||
| Japan | 178.1 | 84.0 | 57.1 | 119.3 | ||||||||||
| Others | 563.2 | 371.1 | 275.1 | 765.2 | ||||||||||
| Total Net Sales | $ | 1,020.1 | $ | 644.2 | $ | 449.5 | $ | 1,159.4 | ||||||
| Operating Margin: | ||||||||||||||
| United States | $ | 111.9 | $ | 79.5 | $ | 47.0 | $ | 116.7 | ||||||
| Japan | 86.3 | 39.5 | 27.9 | 56.5 | ||||||||||
| Others | 225.2 | 157.4 | 119.7 | 335.1 | ||||||||||
| Total Operating Margin | $ | 423.4 | $ | 276.4 | $ | 194.6 | $ | 508.3 | ||||||
| Marketing, distribution and administrative expense* | $ | 354.6 | $ | 207.4 | $ | 135.5 | $ | 401.3 | ||||||
| Merger transaction expense | — | 54.7 | 6.2 | — | ||||||||||
| Interest expense (income), net | (3.4 | ) | (1.4 | ) | 23.9 | 41.5 | ||||||||
| Income before income taxes and minority interest | 72.2 | 15.7 | 29.0 | 65.5 | ||||||||||
| Income taxes | 28.9 | 6.3 | 15.0 | 28.7 | ||||||||||
| Minority Interest | 0.7 | 0.2 | — | — | ||||||||||
| Net Income | $ | 42.6 | $ | 9.2 | $ | 14.0 | $ | 36.8 | ||||||
| Net sales by product line: | ||||||||||||||
| Weight management | $ | 421.9 | $ | 274.4 | $ | 191.2 | $ | 500.1 | ||||||
| Inner nutrition | 443.7 | 280.7 | 195.6 | 505.1 | ||||||||||
| Outer Nutrition® | 106.2 | 64.3 | 44.4 | 105.7 | ||||||||||
| Literature, promotional and other | 48.3 | 24.8 | 18.3 | 48.5 | ||||||||||
| Total Net Sales | $ | 1,020.1 | $ | 644.2 | $ | 449.5 | $ | 1,159.4 | ||||||
| Net sales by geographic region: | ||||||||||||||
| Americas | $ | 386.9 | $ | 257.6 | $ | 166.7 | $ | 424.4 | ||||||
| Europe | 283.1 | 193.7 | 149.0 | 448.2 | ||||||||||
| Asia/Pacific Rim | 172.0 | 108.8 | 76.7 | 167.5 | ||||||||||
| Japan | 178.1 | 84.1 | 57.1 | 119.3 | ||||||||||
| Total Net Sales | $ | 1,020.1 | $ | 644.2 | $ | 449.5 | $ | 1,159.4 | ||||||
| Capital Expenditures: | ||||||||||||||
| United States | $ | 11.5 | $ | 5.4 | $ | 2.6 | $ | 17.3 | ||||||
| Japan | 0.4 | 0.1 | 0.1 | 0.2 | ||||||||||
| Others | 2.9 | 1.3 | 0.9 | 2.9 | ||||||||||
| Total Capital Expenditures | $ | 14.8 | $ | 6.8 | $ | 3.6 | $ | 20.4 | ||||||
F-31
| |
December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
||||||
| |
(Successor) |
(Successor) |
||||||
| Total Assets: | ||||||||
| United States | $ | 566.8 | $ | 601.0 | ||||
| Japan | 104.8 | 62.9 | ||||||
| Others | 184.1 | 240.1 | ||||||
| Total Assets | $ | 855.7 | $ | 904.0 | ||||
| Goodwill: | ||||||||
| United States | $ | 88.4 | $ | 46.0 | ||||
| Japan | 55.5 | 22.7 | ||||||
| Others | 67.2 | 98.8 | ||||||
| Total Goodwill | $ | 211.1 | $ | 167.5 | ||||
12. Derivative Instruments and Hedging Activities
The Company designates certain derivatives as fair value hedges. For all qualifying and highly effective fair value hedges, the changes in the fair value of a derivative and the gain or loss on the hedged asset or liability relating to the risk being hedged are recorded currently in earnings. These amounts are recorded in marketing, distribution, and administrative expenses and provide offsets to one another.
The Company designates certain derivatives as cash flow hedges. The Company engages in a foreign exchange hedging strategy for which the hedged transactions are forecasted foreign currency denominated intercompany transactions. The hedged risk is the variability of the foreign currency where the hedging strategy involves the purchase and sale of average rate options. For the outstanding cash flow hedges on foreign exchange exposures at December 31, 2003, the maximum length of time over which the Company is hedging these exposures is 12 months. The Company also engages in an interest rate hedging strategy for which the hedged transactions are forecasted interest payments on the variable rate term loan. The hedged risk is the variability of interest rate where the hedging strategy involves the purchase of interest rate caps. For all qualifying and highly effective cash flow hedges, the changes in the effective portion of the fair value of the derivative are recorded in other comprehensive income ("OCI"). The ineffective portion of the hedges, which was not material for any periods presented, is recognized in income currently. At December 31, 2003, the net loss in OCI was $1,392,000. Substantially, all OCI amounts will be reclassified to earnings within 12 months.
The Company designates certain derivatives as free standing derivatives for which hedge accounting does not apply. The changes in the fair market value of the derivatives are recorded in the Company's statement of income. The Company purchases average rate put options, which give the Company the right, but not the obligation, to sell foreign currency at a specified exchange rate ("strike rate"). These contracts provide protection in the event the foreign currency weakens beyond the option strike rate. In some instances, the Company sells (writes) foreign currency call options to finance the purchase of put options, which gives the counterparty the right, but not the obligation, to buy foreign currency from the Company at a specified strike rate. These contracts serve to limit the benefit the Company would otherwise derive from strengthening of the foreign currency beyond the strike rate. Such written call options are only entered into contemporaneously with purchased put options. The fair value of option contracts is based on third-party bank quotes.
F-32
The following table provides information about the details of the Company's option contracts. Certain option contracts were designated as cash flow hedges or fair value hedges. Certain option contracts were freestanding derivatives.
| Foreign Currency |
Coverage |
Average strike price |
Fair value |
Maturity date |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
|
(in millions) |
|
|||||||
| At December 31, 2003 | |||||||||||
| Purchase Puts (Company may sell yen/buy USD) | |||||||||||
| Japanese yen | $ | 6.0 | 107.75 - 107.97 | $ | — | Jan - Mar 2004 | |||||
| Japanese yen | 6.0 | 107.39 - 107.62 | 0.1 | Apr - Jun 2004 | |||||||
| Japanese yen | 6.0 | 106.95 - 107.25 | 0.2 | Jul - Sep 2004 | |||||||
| Japanese yen | 6.0 | 106.43 - 106.80 | 0.2 | Oct - Dec 2004 | |||||||
| $ | 24.0 | $ | 0.5 | ||||||||
| Written Calls (Counterparty may buy yen/sell USD) | |||||||||||
| Japanese yen | $ | 6.0 | 102.00 | $ | — | Jan - Mar 2004 | |||||
| Japanese yen | 6.0 | 102.00 | — | Apr - Jun 2004 | |||||||
| Japanese yen | 6.0 | 102.00 | (0.1 | ) | Jul - Sep 2004 | ||||||
| Japanese yen | 6.0 | 102.00 | (0.1 | ) | Oct - Dec 2004 | ||||||
| $ | 24.0 | $ | (0.2 | ) | |||||||
| Purchase Puts (Company may sell euro/buy USD) | |||||||||||
| Euro | $ | 9.4 | 1.1635 - 1.1910 | $ | — | Jan - Mar 2004 | |||||
| Euro | 9.4 | 1.1588 - 1.1881 | 0.1 | Apr - Jun 2004 | |||||||
| Euro | 5.7 | 1.1564 - 1.1579 | — | Jul - Sep 2004 | |||||||
| Euro | 5.7 | 1.150 - 1.1558 | 0.1 | Oct - Dec 2004 | |||||||
| $ | 30.2 | $ | 0.2 | ||||||||
| Written Calls (Counterparty may buy euro/sell USD) | |||||||||||
| Euro | $ | 5.7 | 1.21 | $ | (0.2 | ) | Jan - Mar 2004 | ||||
| Euro | 5.7 | 1.21 | (0.3 | ) | Apr - Jun 2004 | ||||||
| Euro | 5.7 | 1.21 | (0.3 | ) | Jul - Sep 2004 | ||||||
| Euro | 5.7 | 1.21 | (0.3 | ) | Oct - Dec 2004 | ||||||
| $ | 22.8 | $ | (1.1 | ) | |||||||
| At December 31, 2002 | |||||||||||
| Purchase Puts (Company may sell yen/buy USD) | |||||||||||
| Japanese yen | $ | 6.0 | 118.43 - 119.68 | $ | 0.1 | Jan - Mar 2003 | |||||
| Japanese yen | 6.0 | 118.03 - 119.30 | 0.1 | Apr - Jun 2003 | |||||||
| $ | 12.0 | $ | 0.2 | ||||||||
| Purchase Puts (Company may sell euro/buy USD) | |||||||||||
| Euro | $ | 9.0 | 1.0155 - 1.0300 | $ | — | Jan - Mar 2003 | |||||
| Euro | 9.0 | 1.0155 - 1.0300 | $ | 0.2 | Apr - Jun 2003 | ||||||
| $ | 18.0 | $ | 0.2 | ||||||||
Foreign exchange forward contracts are also used to hedge advances between subsidiaries and bank loans denominated in currencies other than their local currency. The objective of these contracts is to neutralize the impact of foreign currency movements on the subsidiary's operating results. The fair value of forward contracts is based on third-party bank quotes.
F-33
The table below describes the forward contracts that were outstanding. All forward contracts were freestanding derivatives.
| Foreign currency |
Contract date |
Forward position |
Maturity date |
Contract rate |
Fair value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
(in millions) |
|
|
(in millions) |
|||||||
| At December 31, 2003 | ||||||||||||
| Buy TWD Sell EURO | 12/02/2003 | $ | 2.6 | 1/5/2004 | 41.1200 | $ | 2.5 | |||||
| Buy CAD Sell EURO | 12/02/2003 | $ | 1.2 | 1/5/2004 | 1.5682 | $ | 1.2 | |||||
| Buy DKK Sell EURO | 12/02/2003 | $ | 0.8 | 1/5/2004 | 7.4410 | $ | 0.8 | |||||
| Buy SEK Sell EURO | 12/02/2003 | $ | 0.8 | 1/5/2004 | 9.0145 | $ | 0.8 | |||||
| Buy AUD Sell EURO | 12/02/2003 | $ | 1.1 | 1/5/2004 | 1.6552 | $ | 1.1 | |||||
| Buy AUD Sell EURO | 12/19/2003 | $ | 1.5 | 1/5/2004 | 1.6810 | $ | 1.5 | |||||
Buy GBP Sell USD |
12/19/2003 |
$ |
3.2 |
1/23/2004 |
1.7636 |
$ |
3.2 |
|||||
| Buy SEK Sell USD | 12/19/2003 | $ | 1.6 | 1/23/2004 | 7.3270 | $ | 1.7 | |||||
| Buy JPY Sell USD | 12/19/2003 | $ | 14.0 | 1/23/2004 | 107.7050 | $ | 14.1 | |||||
| Buy EURO Sell USD | 12/19/2003 | $ | 1.0 | 1/23/2004 | 1.2381 | $ | 1.0 | |||||
At December 31, 2002 |
||||||||||||
| Buy USD Sell Mexican Peso | 12/03/2002 | $ | 10.6 | 1/06/2003 | 10.21 | $ | 10.8 | |||||
| Buy USD Sell Brazilian Real | 12/12/2002 | $ | 1.0 | 1/16/2003 | 3.74 | $ | 0.9 | |||||
All foreign subsidiaries excluding those operating in hyper-inflationary environments designate their local currencies as their functional currency. At year end, the total amount of cash held by foreign subsidiaries primarily in Japan and Korea was $63.4 million of which $3.7 million was maintained or invested in U.S. dollars.
The interest rate cap is used to hedge the interest rate exposure on the term loan which has a variable interest rate. They provide protection in the event the LIBOR rates increase beyond the cap rate. The table below describes the interest rate cap that was outstanding. Interest rate caps were designated as cash flow hedges.
| Interest rate |
Notional amount |
Cap rate |
Fair value |
Maturity date |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
|
(in millions) |
|
||||||
| At December 31, 2003 | ||||||||||
| Interest Rate Cap | $ | 34.4 | 5 | % | $ | — | 10/31/2005 | |||
At December 31, 2002 |
||||||||||
| Interest Rate Cap | $ | 43.8 | 5 | % | $ | 0.1 | 10/31/2005 | |||
F-34
13. Income Taxes
The components of income before income taxes are:
| |
2001 |
2002 |
2002 |
2003 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, |
January 1 to July 31, |
August 1 to December 31, |
Year ended December 31, |
||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
||||||||
| |
(in millions) |
|||||||||||
| Domestic | $ | 50.0 | $ | 3.5 | $ | 8.4 | $ | 14.8 | ||||
| Foreign | 22.2 | 12.2 | 20.6 | 50.7 | ||||||||
| $ | 72.2 | $ | 15.7 | $ | 29.0 | $ | 65.5 | |||||
Income taxes are as follows:
| |
2001 |
2002 |
2002 |
2003 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, |
January 1 to July 31, |
August 1 to December 31, |
Year ended December 31, |
|||||||||
| |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
|||||||||
| |
(in millions) |
||||||||||||
| Current: | |||||||||||||
| Foreign | $ | 17.7 | $ | 7.3 | $ | 17.7 | $ | 24.7 | |||||
| Federal | 12.0 | (1.9 | ) | (4.5 | ) | 14.5 | |||||||
| State | 2.1 | 0.4 | 0.7 | 1.7 | |||||||||
Deferred: |
|||||||||||||
| Foreign | 3.7 | (0.5 | ) | (1.4 | ) | (4.3 | ) | ||||||
| Federal | (6.5 | ) | 1.1 | 2.7 | (8.2 | ) | |||||||
| State | (0.1 | ) | (0.1 | ) | (0.2 | ) | 0.3 | ||||||
| $ | 28.9 | $ | 6.3 | $ | 15.0 | $ | 28.7 | ||||||
F-35
The tax effects of temporary differences which gave rise to deferred income tax assets and liabilities are as follows:
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2002 |
2003 |
|||||
| Deferred income tax assets: | |||||||
| Accruals not currently deductible | $ | 13.5 | $ | 17.4 | |||
| Accrued foreign withholding tax on unremitted earnings | 2.8 | 2.8 | |||||
| Foreign tax credits and tax loss carryforwards of certain foreign subsidiaries | 24.8 | 43.9 | |||||
| Depreciation/amortization | 0.7 | 0.1 | |||||
| Deferred compensation plan | 13.1 | 9.1 | |||||
| Accrued state income taxes | — | 0.6 | |||||
| Accrued vacation | 1.6 | 1.4 | |||||
| Unrealized foreign exchange | 6.2 | 4.7 | |||||
| Other | 1.6 | 3.2 | |||||
| Gross deferred income tax assets | $ | 64.3 | $ | 83.2 | |||
| Less: valuation allowance | (20.1 | ) | (42.5 | ) | |||
| Net deferred income tax assets | 44.2 | 40.7 | |||||
Deferred income tax liabilities: |
|||||||
| Intangible assets | $ | 126.3 | $ | 140.2 | |||
| Inventory deductibles | 1.9 | 3.3 | |||||
| $ | 128.2 | 143.5 | |||||
| Net | $ | (84.0 | ) | $ | (102.8 | ) | |
At December 31, 2003, the Company's deferred income tax asset for U.S. foreign tax credits of $43.6 million and net operating loss carryforwards of certain foreign subsidiaries of $3.1 million was reduced by a valuation allowance of $42.5 million. The valuation allowance includes an increase during 2003 of $14.7 million attributable to U.S. foreign tax credits applicable to periods prior to the Merger, therefore this amount has not affected the 2003 income statement. If tax benefits are recognized in the future through reduction of the valuation allowance, $32.9 million of such benefits will be allocated to reduce goodwill.
During 2002, the Company recorded a deferred income tax liability in connection with identified intangible assets recorded in the acquisition of the Company. During 2003, in connection with the revaluation of these assets, the associated deferred income tax liability was increased by $28.3 million.
During 2003, the Company recorded a deferred tax liability of $2.7 million in connection with the recording of other comprehensive income for the year related to currency translation. The total deferred tax liability at December 31, 2003 relating to accumulated comprehensive income was $2.3 million.
The net operating loss carryforwards expire in varying amounts between 2004 and 2012. The foreign tax credit carryforwards expire in varying amounts between 2005 and 2008. Realization of the income tax carryforwards is dependent on generating sufficient taxable income prior to expiration of the carryforwards. Although realization is not assured, management believes it is more likely than not that the net carrying value of the income tax carryforwards will be realized. The amount of the income tax carryforwards that is considered realizable, however, could be reduced if estimates of future taxable income during the carryforward period are reduced.
F-36
Deferred income taxes of $2.8 million have been provided on the undistributed earnings of non-U.S. subsidiaries that are not expected to be permanently reinvested in such subsidiaries.
The tax expense differs from the "expected" income tax expense by applying the United States statutory rate of 35% as follows:
| |
Year ended December 31, 2001 |
January 1 to July 31, 2002 |
August 1 to December 31, 2002 |
Year ended December 31, 2003 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(predecessor) |
(predecessor) |
(successor) |
(successor) |
|||||||||||
| |
(in millions) |
||||||||||||||
| Tax expense at United States statutory rate | $ | 25.3 | $ | 5.5 | $ | 10.1 | $ | 22.9 | |||||||
| Increase (decrease) in tax resulting from: | |||||||||||||||
| Differences between U.S. and foreign tax rates on foreign income, including withholding taxes | 7.1 | 1.8 | 7.4 | 3.9 | |||||||||||
| U.S. tax (benefit) on foreign income and foreign tax credits | (7.9 | ) | (1.6 | ) | (5.4 | ) | (6.3 | ) | |||||||
| Increase (decrease) in valuation allowances | 2.3 | 0.1 | 1.7 | 7.7 | |||||||||||
| State taxes, net of federal benefit | 1.2 | 0.4 | 0.8 | 1.3 | |||||||||||
| Other | 0.9 | 0.1 | 0.4 | (0.8 | ) | ||||||||||
| Total | $ | 28.9 | $ | 6.3 | $ | 15.0 | $ | 28.7 | |||||||
The U.S. tax benefit on foreign income and foreign tax credits shown above resulted in increases to the deferred tax asset for foreign tax credit carryovers and the valuation allowance. The valuation allowance for deferred tax assets applicable to periods prior to the Merger was also adjusted, as discussed above.
14. Restructuring Reserve
As of the date of the Merger, the Company began to assess and formulate a plan to reduce costs of the business and recorded a severance and restructuring accrual as part of the cost of the Merger. The accrued severance is for employees including executives, corporate functions, and administrative support that were indentified at the time of the Merger. Actions required by the plan of termination began immediately after consummation of the transaction. The remaining balance of the restructuring reserve at December 31, 2003 of $2.5 million represents scheduled severance payments for employees.
The following table summarizes the activity in the Company's restructuring accrual subsequent to July 31, 2002:
| |
(in millions) |
|||
|---|---|---|---|---|
| Accrual made as of July 31, 2002 | $ | 10.2 | ||
| Additional accrual | 3.0 | |||
| Payments made | (10.7 | ) | ||
| Balance at December 31, 2003 | $ | 2.5 | ||
F-37
15. Supplemental Information
The consolidated financial statement data, as of December 31, 2003 and 2002, for the year ended December 31, 2003 and for the period from inception to December 31, 2002 has been aggregated by entities that guarantee the Senior Subordinated Notes (the "Guarantors") and entities that do not guarantee the Senior Subordinated Notes (the "Non-Guarantors"). The Guarantors include WH Intermediate, Lux Holdings, Lux Intermediate, Lux CM (collectively, the "Parent Guarantors") and Herbalife's operating subsidiaries in Brazil, Finland, Israel, Japan, Mexico, United Kingdom, U.S. (other than Herbalife Investment Co., LLC), Sweden, Taiwan and Thailand (collectively, the "Subsidiary Guarantors"). All other subsidiaries are Non-Guarantors.
Consolidating condensed statements of income for year ended December 31, 2003 and the periods from January 1 to July 31, 2002, August 1 to December 31, 2002, and the year ended December 31, 2001 are summarized as follows: (in millions)
| |
Year Ended December 31, 2003 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Successor) |
Herbalife International, Inc. |
Parent guarantors |
Subsidiary guarantors |
WH Holdings (Cayman Islands) Ltd. non-guarantor |
Non- guarantors |
Eliminations |
Total consolidated |
||||||||||||||
| Net sales | $ | — | $ | 126.4 | $ | 916.8 | $ | — | $ | 273.2 | $ | (157.0 | ) | $ | 1,159.4 | ||||||
| Cost of sales | — | 25.2 | 232.1 | — | 134.6 | (156.1 | ) | 235.8 | |||||||||||||
| Royalty overrides | — | 4.1 | 249.2 | — | 162.1 | — | 415.4 | ||||||||||||||
| Marketing, distribution & administrative expenses | 40.3 | 6.0 | 272.7 | 0.4 | 81.8 | — | 401.2 | ||||||||||||||
| Equity in subsidiary (income) loss | (76.6 | ) | (42.9 | ) | (2.1 | ) | (43.6 | ) | — | 165.2 | — | ||||||||||
| Interest expense—net | 34.9 | 0.2 | (0.1 | ) | 6.4 | 0.1 | — | 41.5 | |||||||||||||
| Intercompany charges | (25.1 | ) | 90.2 | 67.2 | — | (132.3 | ) | — | — | ||||||||||||
| Income before income taxes and minority interest | 26.5 | 43.6 | 97.8 | 36.8 | 26.9 | (166.1 | ) | 65.5 | |||||||||||||
| Income taxes | (16.2 | ) | — | 35.9 | — | 9.2 | (0.2 | ) | 28.7 | ||||||||||||
| NET INCOME | $ | 42.7 | $ | 43.6 | $ | 61.9 | $ | 36.8 | $ | 17.7 | $ | (165.9 | ) | $ | 36.8 | ||||||
| |
August 1 to December 31, 2002 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Successor) |
Herbalife International, Inc. |
Parent guarantors |
Subsidiary guarantors |
WH Holdings (Cayman Islands) Ltd. non-guarantor |
Non- guarantors |
Eliminations |
Total consolidated |
||||||||||||||
| Net sales | $ | — | $ | — | $ | 386.1 | $ | — | $ | 101.3 | $ | (37.9 | ) | $ | 449.5 | ||||||
| Cost of sales | — | — | 86.8 | — | 46.5 | (38.3 | ) | 95.0 | |||||||||||||
| Royalty overrides | — | — | 103.9 | — | 56.0 | — | 159.9 | ||||||||||||||
| Marketing, distribution & administrative expenses | 4.1 | — | 95.7 | 0.2 | 35.5 | — | 135.5 | ||||||||||||||
| Merger transaction expenses | — | — | — | 6.2 | — | — | 6.2 | ||||||||||||||
| Equity in subsidiary (income) loss | (37.1 | ) | (32.0 | ) | (0.3 | ) | (22.9 | ) | — | 92.3 | — | ||||||||||
| Interest expense—net | 22.6 | — | (1.1 | ) | 2.4 | — | — | 23.9 | |||||||||||||
| Intercompany charges | (4.8 | ) | — | 45.5 | — | (41.0 | ) | 0.3 | — | ||||||||||||
| Income before income taxes and minority interest | 15.2 | 32.0 | 55.6 | 14.1 | 4.3 | (92.2 | ) | 29.0 | |||||||||||||
| Income taxes | (16.7 | ) | 9.1 | 21.2 | (0.1 | ) | 1.5 | — | 15.0 | ||||||||||||
| Income before minority interest | 31.9 | 22.9 | 34.4 | 14.2 | 2.8 | (92.2 | ) | 14.0 | |||||||||||||
| Minority interest | — | — | — | — | — | — | — | ||||||||||||||
| NET INCOME | $ | 31.9 | $ | 22.9 | $ | 34.4 | $ | 14.2 | $ | 2.8 | $ | (92.2 | ) | $ | 14.0 | ||||||
F-38
| |
January 1 to July 31, 2002 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Predecessor) |
Herbalife International, Inc. |
Subsidiary guarantors |
Non- guarantors |
Eliminations |
Total consolidated |
|||||||||||
| Net sales | $ | — | $ | 551.3 | $ | 142.5 | $ | (49.6 | ) | $ | 644.2 | |||||
| Cost of sales | — | 128.1 | 63.2 | (50.7 | ) | 140.6 | ||||||||||
| Royalty overrides | — | 147.3 | 79.9 | — | 227.2 | |||||||||||
| Marketing, distribution & administrative expenses | (0.8 | ) | 165.9 | 42.3 | — | 207.4 | ||||||||||
| Merger transaction expenses | 54.7 | — | — | — | 54.7 | |||||||||||
| Equity in subsidiary (income) loss | (36.4 | ) | (0.5 | ) | — | 36.9 | — | |||||||||
| Interest expense—net | — | (1.8 | ) | 0.4 | — | (1.4 | ) | |||||||||
| Intercompany charges | (7.5 | ) | 62.9 | (55.4 | ) | — | — | |||||||||
| Income before income taxes and minority interest | (10.0 | ) | 49.4 | 12.1 | (35.8 | ) | 15.7 | |||||||||
| Income taxes | (18.6 | ) | 19.9 | 5.0 | — | 6.3 | ||||||||||
| Income before minority interest | 8.6 | 29.5 | 7.1 | (35.8 | ) | 9.4 | ||||||||||
| Minority interest | — | 0.2 | — | — | 0.2 | |||||||||||
| NET INCOME | $ | 8.6 | $ | 29.3 | $ | 7.1 | $ | (35.8 | ) | $ | 9.2 | |||||
| |
Year Ended December 31, 2001 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Predecessor) |
Herbalife International, Inc. |
Subsidiary guarantors |
Non- guarantors |
Eliminations |
Total consolidated |
|||||||||||
| Net sales | $ | — | $ | 877.8 | $ | 218.3 | $ | (76.0 | ) | $ | 1,020.1 | |||||
| Cost of sales | — | 216.9 | 97.0 | (72.4 | ) | 241.5 | ||||||||||
| Royalty overrides | — | 238.8 | 116.4 | — | 355.2 | |||||||||||
| Marketing, distribution & administrative expenses | 0.4 | 281.2 | 73.0 | — | 354.6 | |||||||||||
| Merger transaction expenses | — | — | — | — | — | |||||||||||
| Equity in subsidiary (income) loss | (43.4 | ) | (0.8 | ) | — | 44.2 | — | |||||||||
| Interest expense—net | — | (3.4 | ) | — | — | (3.4 | ) | |||||||||
| Intercompany charges | (6.4 | ) | 76.5 | (70.0 | ) | (0.1 | ) | — | ||||||||
| Income before income taxes and minority interest | 49.4 | 68.6 | 1.9 | (47.7 | ) | 72.2 | ||||||||||
| Income taxes | 2.5 | 22.6 | 3.8 | — | 28.9 | |||||||||||
| Income before minority interest | 46.9 | 46.0 | (1.9 | ) | (47.7 | ) | 43.3 | |||||||||
| Minority interest | — | 0.7 | — | — | 0.7 | |||||||||||
| NET INCOME | $ | 46.9 | $ | 45.3 | $ | (1.9 | ) | $ | (47.7 | ) | $ | 42.6 | ||||
F-39
Consolidating condensed balance sheet data as of December 31, 2003 and as of December 31, 2002 is summarized as follows: (in millions)
| |
December 31, 2003 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Herbalife International, Inc. |
Parent guarantors |
Subsidiary guarantors |
WH Holdings (Cayman Islands) Ltd. Non- guarantor |
Non-guarantors |
Eliminations |
Total consolidated |
|||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||||||
| Cash and marketable securities | $ | 0.1 | $ | 13.8 | $ | 92.5 | $ | 9.4 | $ | 40.6 | $ | — | $ | 156.4 | ||||||||
| Receivables | — | — | 23.0 | 1.5 | 7.5 | — | 32.0 | |||||||||||||||
| Intercompany receivables | 196.7 | (23.3 | ) | (89.4 | ) | — | (84.0 | ) | — | — | ||||||||||||
| Inventories | — | 26.0 | 23.9 | — | 15.0 | (5.5 | ) | 59.4 | ||||||||||||||
| Other current assets | (2.5 | ) | 2.2 | 26.9 | — | 3.4 | — | 30.0 | ||||||||||||||
| Total current assets | 194.3 | 18.7 | 76.9 | 10.9 | (17.5 | ) | (5.5 | ) | 277.8 | |||||||||||||
| Property, net | 0.3 | 2.1 | 37.7 | — | 5.3 | — | 45.4 | |||||||||||||||
OTHER NON-CURRENT ASSETS |
448.9 |
65.8 |
129.8 |
238.7 |
68.5 |
(370.9 |
) |
580.8 |
||||||||||||||
TOTAL ASSETS |
$ |
643.5 |
$ |
86.6 |
$ |
244.4 |
$ |
249.6 |
$ |
56.3 |
$ |
(376.4 |
) |
$ |
904.0 |
|||||||
CURRENT LIABILITIES: |
||||||||||||||||||||||
| Accounts payable | $ | — | $ | 8.2 | $ | 10.4 | $ | 0.1 | $ | 3.8 | $ | — | $ | 22.5 | ||||||||
| Royalties overrides | — | 0.7 | 45.7 | — | 30.1 | — | 76.5 | |||||||||||||||
| Accrued compensation and expenses | 8.7 | 10.2 | 44.7 | — | 15.2 | — | 78.8 | |||||||||||||||
| Other current liabilities | 41.1 | 0.4 | 55.6 | (0.2 | ) | 1.5 | — | 98.4 | ||||||||||||||
| Total current liabilities | 49.8 | 19.5 | 156.4 | (0.1 | ) | 50.6 | — | 276.2 | ||||||||||||||
| NON-CURRENT LIABILITIES | 351.9 | 0.3 | (0.9 | ) | 38.0 | 0.7 | — | 390.0 | ||||||||||||||
| MINORITY INTEREST | — | — | — | — | — | — | — | |||||||||||||||
| SHAREHOLDERS' EQUITY | 241.8 | 66.8 | 88.9 | 211.7 | 5.0 | (376.4 | ) | 237.8 | ||||||||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | $ | 643.5 | $ | 86.6 | $ | 244.4 | $ | 249.6 | $ | 56.3 | $ | (376.4 | ) | $ | 904.0 | |||||||
F-40
| |
December 31, 2002 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Herbalife International, Inc. |
Parent guarantors |
Subsidiary guarantors |
WH Holdings (Cayman Islands) Ltd. Non- guarantor |
Non-guarantors |
Eliminations |
Total consolidated |
|||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||||||
| Cash and marketable securities | $ | 0.3 | $ | — | $ | 39.6 | $ | 10.6 | $ | 25.5 | $ | — | $ | 76.0 | ||||||||
| Receivables | — | — | 24.0 | 0.2 | 5.7 | (0.9 | ) | 29.0 | ||||||||||||||
| Intercompany receivables | 141.8 | — | (75.9 | ) | — | (65.9 | ) | — | — | |||||||||||||
| Inventories | — | — | 46.2 | — | 15.2 | (4.5 | ) | 56.9 | ||||||||||||||
| Other current assets | 0.2 | 0.2 | 38.2 | — | 18.6 | (14.4 | ) | 42.8 | ||||||||||||||
Total current assets |
142.3 |
0.2 |
72.1 |
10.8 |
(0.9 |
) |
(19.8 |
) |
204.7 |
|||||||||||||
| Property, net | — | — | 37.4 | — | 8.7 | — | 46.1 | |||||||||||||||
OTHER NON-CURRENT ASSETS |
394.3 |
32.0 |
195.3 |
218.0 |
55.2 |
(289.9 |
) |
604.9 |
||||||||||||||
TOTAL ASSETS |
$ |
536.6 |
$ |
32.2 |
$ |
304.8 |
$ |
228.8 |
$ |
63.0 |
$ |
(309.7 |
) |
$ |
855.7 |
|||||||
CURRENT LIABILITIES: |
||||||||||||||||||||||
| Accounts payable | $ | — | $ | — | $ | 16.9 | $ | 0.7 | $ | 4.9 | $ | (0.9 | ) | $ | 21.6 | |||||||
| Royalties overrides | — | — | 46.5 | — | 22.6 | — | 69.1 | |||||||||||||||
Accrued compensation and expenses |
12.1 |
— |
42.7 |
— |
15.0 |
— |
69.8 |
|||||||||||||||
| Other current liabilities | (14.8 | ) | 9.3 | 41.7 | (0.2 | ) | 15.6 | (14.6 | ) | 37.0 | ||||||||||||
| Total current liabilities | (2.7 | ) | 9.3 | 147.8 | 0.5 | 58.1 | (15.5 | ) | 197.5 | |||||||||||||
| NON-CURRENT LIABILITIES | 409.1 | — | 19.7 | 36.7 | 1.4 | — | 466.9 | |||||||||||||||
| MINORITY INTEREST | — | — | — | — | — | — | — | |||||||||||||||
| SHAREHOLDERS' EQUITY | 130.2 | 22.9 | 137.3 | 191.6 | 3.5 | (294.2 | ) | 191.3 | ||||||||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | $ | 536.6 | $ | 32.2 | $ | 304.8 | $ | 228.8 | $ | 63.0 | $ | (309.7 | ) | $ | 855.7 | |||||||
F-41
Consolidating condensed statement of cash flows data year ended December 31, 2003, the periods of January 1 to July 31, 2002 and August 1 to December 31, 2002, and the year ended December 31, 2001 is summarized as follows: (in millions)
| |
December 31, 2003 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Successor) |
Herbalife International, Inc. |
Parent guarantors |
Subsidiary guarantors |
WH Holdings (Cayman Islands) Ltd. Non-guarantor |
Non- guarantors |
Eliminations |
Total consolidated |
|||||||||||||||
| Net cash provided by (used in) operating activities | $ | 43.5 | $ | 57.0 | $ | 98.7 | $ | 37.3 | $ | 32.7 | $ | (174.6 | ) | $ | 94.6 | |||||||
| Net cash provided by (used in) investing activities | (22.8 | ) | (45.9 | ) | 1.5 | (38.9 | ) | (2.7 | ) | 111.7 | 2.9 | |||||||||||
| Net cash provided by (used in) financing activities | (21.0 | ) | — | (48.5 | ) | 5.3 | (17.5 | ) | 62.9 | (18.8 | ) | |||||||||||
| Effect of exchange rate changes on cash | — | 2.6 | 2.6 | — | 2.6 | — | 7.8 | |||||||||||||||
| Cash at beginning of period | 0.5 | — | 38.2 | — | 25.5 | — | 64.2 | |||||||||||||||
| Cash at end of period | $ | 0.2 | $ | 13.7 | $ | 92.5 | $ | 3.7 | $ | 40.6 | $ | — | $ | 150.7 | ||||||||
| |
August 1 to December 31, 2002 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Successor) |
Herbalife International, Inc. |
Parent guarantors |
Subsidiary guarantors |
WH Holdings (Cayman Islands) Ltd. Non-guarantor |
Non- guarantors |
Eliminations |
Total consolidated |
|||||||||||||||
| Net cash provided by (used in) operating activities | $ | 208.3 | $ | 32.0 | $ | (136.3 | ) | $ | (8.3 | ) | $ | 7.5 | $ | (75.1 | ) | $ | 28.1 | |||||
| Net cash provided by (used in) investing activities | (684.8 | ) | (32.0 | ) | 181.8 | (203.2 | ) | 22.7 | 259.5 | (456.0 | ) | |||||||||||
| Net cash provided by (used in) financing activities | 477.0 | — | (7.4 | ) | 211.5 | (5.2 | ) | (184.4 | ) | 491.5 | ||||||||||||
| Effect of exchange rate changes on cash | — | — | 0.1 | — | 0.5 | — | 0.6 | |||||||||||||||
| Cash at beginning of period | — | — | — | — | — | — | — | |||||||||||||||
| Cash at end of period | $ | 0.5 | $ | — | $ | 38.2 | $ | — | $ | 25.5 | $ | — | $ | 64.2 | ||||||||
| |
January 1 to July 31, 2002 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Predecessor) |
Herbalife International, Inc. |
Subsidiary guarantors |
Non- guarantors |
Eliminations |
Total consolidated |
|||||||||||
| Net cash provided by (used in) operating activities | $ | 32.0 | $ | 46.9 | $ | (2.1 | ) | $ | (38.9 | ) | $ | 37.9 | ||||
| Net cash provided by (used in) investing activities | (10.5 | ) | 26.9 | 1.3 | 1.3 | 19.0 | ||||||||||
| Net cash provided by (used in) financing activities | (21.5 | ) | (40.4 | ) | (11.0 | ) | 37.6 | (35.3 | ) | |||||||
| Effect of exchange rate changes on cash | — | (0.6 | ) | 1.6 | — | 1.0 | ||||||||||
| Cash at beginning of period | 0.2 | 145.3 | 33.7 | — | 179.2 | |||||||||||
| Cash at end of period | $ | 0.2 | $ | 178.1 | $ | 23.5 | $ | — | $ | 201.8 | ||||||
F-42
| |
Year Ended December 31, 2001 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Herbalife International, Inc. |
Subsidiary guarantors |
Non- guarantors |
Eliminations |
Total consolidated |
|||||||||||
| Net cash provided by (used in) operating activities | $ | 46.3 | $ | 110.6 | $ | 10.1 | $ | (71.5 | ) | $ | 95.5 | |||||
| Net cash provided by (used in) investing activities | (45.1 | ) | 89.8 | (2.4 | ) | (58.7 | ) | (16.4 | ) | |||||||
| Net cash provided by (used in) financing activities | (1.2 | ) | (120.8 | ) | (11.7 | ) | 130.2 | (3.5 | ) | |||||||
| Effect of exchange rate changes on cash | — | (4.5 | ) | (2.2 | ) | — | (6.7 | ) | ||||||||
| Cash at beginning of period | 0.2 | 70.2 | 39.9 | — | 110.3 | |||||||||||
| Cash at end of period | $ | 0.2 | $ | 145.3 | $ | 33.7 | $ | — | $ | 179.2 | ||||||
16. Quarterly Information (Unaudited)
| |
2002 |
2003 |
||||
|---|---|---|---|---|---|---|
| |
|
(Successor) |
||||
| First Quarter Ended March 31 | ||||||
| Net sales | $ | 265.8 | $ | 280.0 | ||
| Gross profit | 208.7 | 223.1 | ||||
| Net income | 19.9 | 16.9 | ||||
Second Quarter Ended June 30 |
||||||
| Net sales | $ | 282.0 | $ | 288.9 | ||
| Gross profit | 219.3 | 230.5 | ||||
| Net income | 13.4 | 17.2 | ||||
Third Quarter Ended September 30 |
||||||
| Net sales | $ | 290.4 | ||||
| Gross profit | 231.4 | |||||
| Net income | 1.7 | |||||
July 1 To July 31 (Predecessor) |
||||||
| Net sales | $ | 96.4 | ||||
| Gross profit | 75.7 | |||||
| Net loss | (24.1 | ) | ||||
August 1 To September 30 (Successor) |
||||||
| Net sales | $ | 176.2 | ||||
| Gross profit | 138.0 | |||||
| Net loss | (1.2 | ) | ||||
Fourth Quarter Ended December 31 (Successor) |
||||||
| Net sales | $ | 273.3 | $ | 300.1 | ||
| Gross profit | 216.5 | 238.7 | ||||
| Net income | 15.2 | 1.1 | ||||
17. Subsequent Events
On March 8, 2004, Holdings and its wholly-owned subsidiary WH Capital Corporation completed a $275 million offering of 9.5% Notes due 2011 (the "Notes"). The proceeds of the offering together with available cash were used to pay the cash redemption price due upon redemption of all outstanding Holdings convertible preferred shares, including all accrued and unpaid dividends, to redeem Holdings' 15.5% Senior Notes and to pay related fees and expenses. Interest on the notes will be paid in cash semi-annually in arrear on April 1 and October 1 of each year, starting on October 1, 2004. The Notes are Holdings' general unsecured obligations, ranking equally with any of the existing and future senior indebtedness and senior to all of Holdings' future subordinated indebtedness. Also, the Notes are effectively subordinated to all existing and future indebtedness and other liabilities of Holding's subsidiaries.
F-43
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED BALANCE SHEETS
| |
December 31, 2003 |
March 31, 2004 |
|||||
|---|---|---|---|---|---|---|---|
| |
|
(unaudited) |
|||||
| CURRENT ASSETS: | |||||||
| Cash and cash equivalents | $ | 150,679,000 | $ | 123,002,000 | |||
| Restricted cash | 5,701,000 | — | |||||
| Receivables net allowance for doubtful accounts of $5,690,000 (2004) and $2,527,000 (2003), including related party receivables of $323,000 (2004 and 2003) | 31,977,000 | 33,775,000 | |||||
| Inventories | 59,397,000 | 64,134,000 | |||||
| Prepaid expenses and other current assets | 20,825,000 | 24,734,000 | |||||
| Deferred income taxes | 9,164,000 | 8,672,000 | |||||
| Total current assets | 277,743,000 | 254,317,000 | |||||
Property, at cost, net of accumulated depreciation and amortization of $19,696,000 (2004) and $17,607,000 (2003) |
45,411,000 |
45,744,000 |
|||||
| Deferred compensation plan assets | 21,340,000 | 21,425,000 | |||||
| Other assets | 5,795,000 | 5,961,000 | |||||
| Deferred financing costs, net of accumulated amortization of $12,262,000 (2004) and $10,266,000 (2003) | 33,278,000 | 31,464,000 | |||||
| Marketing franchise | 310,000,000 | 310,000,000 | |||||
| Distributor Network, net of accumulated amortization of $31,222,000 (2004) and $26,539,000 (2003) | 29,661,000 | 24,978,000 | |||||
| Product certification, product formulae and other intangible assets, net of accumulated amortization of $11,166,000 (2004) and $9,491,000 (2003) | 13,219,000 | 11,610,000 | |||||
| Goodwill | 167,517,000 | 167,517,000 | |||||
| TOTAL | $ | 903,964,000 | $ | 873,016,000 | |||
LIABILITIES AND STOCKHOLDER'S EQUITY |
|||||||
| CURRENT LIABILITIES: | |||||||
| Accounts payable | $ | 22,526,000 | $ | 23,238,000 | |||
| Royalty overrides | 76,522,000 | 68,496,000 | |||||
| Accrued compensation | 19,127,000 | 16,592,000 | |||||
| Accrued expenses | 59,669,000 | 64,596,000 | |||||
| Current portion of long term debt | 72,377,000 | 23,465,000 | |||||
| Advance sales deposits | 6,574,000 | 12,550,000 | |||||
| Income taxes payable | 19,427,000 | 26,591,000 | |||||
| Total current liabilities | $ | 276,222,000 | $ | 235,528,000 | |||
NON-CURRENT LIABILITIES: |
|||||||
| Long term debt, net of current portion, including related party debt of $6,400,000 (2004) and $23,700,000 (2003) | 252,917,000 | 487,157,000 | |||||
| Deferred compensation | 22,442,000 | 22,327,000 | |||||
| Deferred income taxes | 111,910,000 | 108,667,000 | |||||
| Other non-current liabilities | 2,685,000 | 2,561,000 | |||||
| Total liabilities | $ | 666,176,000 | $ | 856,240,000 | |||
| COMMITMENTS AND CONTINGENCIES | — | — | |||||
STOCKHOLDER'S EQUITY: |
|||||||
| Preferred shares, $0.01 par value (aggregate liquidation preference $446,241,000 (2003), 12% Series A cumulative and convertible, 106,000,000 (2004) and (2003) shares authorized, 0 (2004) and 102,013,572 (2003) shares issued and outstanding | 102,000 | — | |||||
| Common stock, $0.001 par value, 250,000,000 shares authorized, 104,054,388 shares issued and outstanding (2004) | — | 104,000 | |||||
| Paid-in-capital in excess of par value | 183,407,000 | 744,000 | |||||
| Accumulated other comprehensive income (loss) | 3,427,000 | 4,061,000 | |||||
| Retained earnings | 50,852,000 | 11,867,000 | |||||
| Total shareholders' equity | 237,788,000 | 16,776,000 | |||||
| TOTAL | $ | 903,964,000 | $ | 873,016,000 | |||
See the accompanying notes to consolidated financial statements
F-44
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED STATEMENTS OF INCOME
| |
Three Months Ended |
||||||
|---|---|---|---|---|---|---|---|
| |
March 31 2003 |
March 31 2004 |
|||||
| |
(Unaudited) |
||||||
| Product Sales | $ | 240,398,000 | $ | 278,139,000 | |||
| Handling & freight income | 39,641,000 | 45,914,000 | |||||
| Net sales | 280,039,000 | 324,053,000 | |||||
| Cost of sales | 56,960,000 | 63,618,000 | |||||
| Gross profit | 223,079,000 | 260,435,000 | |||||
| Royalty overrides | 99,511,000 | 115,857,000 | |||||
| Marketing, distribution & administrative expenses (included $1,800,000 and $3,100,000 of related party expenses for the three months ended March 31, 2004 and 2003, respectively) | 84,376,000 | 107,842,000 | |||||
| Interest expense—net | 9,948,000 | 27,372,000 | |||||
| Income before income taxes | 29,244,000 | 9,364,000 | |||||
| Income taxes | 12,374,000 | 9,849,000 | |||||
| NET INCOME (LOSS) | $ | 16,870,000 | $ | (485,000 | ) | ||
See the accompanying notes to consolidated financial statements
F-45
WH HOLDINGS (CAYMAN ISLANDS) LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
Three Months Ended |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
March 31, 2003 |
March 31, 2004 |
||||||
| |
(Unaudited) |
|||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net income | $ | 16,870,000 | $ | (485,000 | ) | |||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 6,389,000 | 11,406,000 | ||||||
| Amortization of discount and deferred financing costs | 2,045,000 | 2,152,000 | ||||||
| Deferred income taxes | (614,000 | ) | (2,651,000 | ) | ||||
| Unrealized foreign exchange loss (gain) | 1,804,000 | (124,000 | ) | |||||
| Loss on repurchase of senior notes | 0 | 15,435,000 | ||||||
| Other | 36,000 | 406,000 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Receivables | (4,619,000 | ) | (1,874,000 | ) | ||||
| Inventories | 557,000 | (5,315,000 | ) | |||||
| Prepaid expenses and other current assets | 2,579,000 | (2,795,000 | ) | |||||
| Accounts payable | (3,158,000 | ) | 780,000 | |||||
| Royalty overrides | (11,101,000 | ) | (7,796,000 | ) | ||||
| Accrued expenses and accrued compensation | (14,229,000 | ) | 3,303,000 | |||||
| Advance sales deposits | 3,067,000 | 6,041,000 | ||||||
| Income taxes payable | 5,231,000 | 7,352,000 | ||||||
| Deferred compensation liability | (8,363,000 | ) | (115,000 | ) | ||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | (3,506,000 | ) | 25,720,000 | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchases of property | (1,349,000 | ) | (4,575,000 | ) | ||||
| Proceeds from sale of property | 1,000 | 1,000 | ||||||
| Changes in restricted cash | 1,196,000 | 5,701,000 | ||||||
| Changes in marketable securities, net | 6,000 | 0 | ||||||
| Changes in other assets | (76,000 | ) | (2,591,000 | ) | ||||
| Deferred compensation plan assets | 11,304,000 | (85,000 | ) | |||||
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | $ | 11,082,000 | $ | (1,549,000 | ) | |||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Additions to bank loans and contract payables | 295,000 | 369,000 | ||||||
| Principal payments on bank loans and contract payables | (2,354,000 | ) | (45,387,000 | ) | ||||
| Issuance of notes | 289,000 | 267,437,000 | ||||||
| Repurchase of senior notes | 0 | (51,002,000 | ) | |||||
| Redemption of preferred stock | 0 | (183,115,000 | ) | |||||
| Dividends paid on preferred stock | 0 | (38,500,000 | ) | |||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | (1,770,000 | ) | (50,198,000 | ) | ||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH | 93,000 | (1,650,000 | ) | |||||
| NET CHANGE IN CASH AND CASH EQUIVALENTS | 5,899,000 | (27,677,000 | ) | |||||
| CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | 64,201,000 | 150,679,000 | ||||||
| CASH AND CASH EQUIVALENTS, END OF PERIOD | $ | 70,100,000 | $ | 123,002,000 | ||||
| NON-CASH ACTIVITIES: | ||||||||
| Acquisitions of property from capital leases | $ | 1,371,000 | $ | 847,000 | ||||
See the accompanying notes to consolidated financial statements
F-46
WH HOLDINGS (CAYMAN ISLANDS) LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION
WH Holdings (Cayman Islands) Ltd, a Cayman Islands exempted limited liability company ("Holdings"), incorporated on April 4, 2002, and its direct and indirect wholly owned subsidiaries, WH Intermediate Holdings Ltd, a Cayman Island company (WH Intermediate"), WH Luxembourg Holdings S.à.R.L., a Luxembourg unipersonal limited liability company ("Lux Holdings"), WH Luxembourg Intermediate Holdings S.à.R.L., a Luxembourg unipersonal limited liability company ("Lux Intermediate"), Herbalife International Luxembourg S.à.R.L. ("Herbalife Lux"), formerly known as WH Luxembourg CM S.à.R.L., a Luxembourg unipersonal limited liability, and WH Acquisition Corp., a Nevada corporation ("WH Acquisition"), were formed on behalf of Whitney & Co., LLC ("Whitney") and Golden Gate Private Equity, Inc. ("Golden Gate"), in order to acquire Herbalife International, Inc., a Nevada corporation, and its subsidiaries ("Herbalife" or "Predecessor") on July 31, 2002 (the "Merger"). Holdings and its subsidiaries are referred to collectively herein as the Company. Holdings' 12% Series A Cumulative Convertible Preferred Shares are referred to as "preferred shares" and Holdings' Common Shares are referred to as "common shares."
2. BASIS OF PRESENTATION
The unaudited interim financial information of the Company has been prepared in accordance with Article 10 of the Securities and Exchange Commission's Regulation S-X. The Company's financial statements as of March 31, 2004 include WH Intermediate, and all of its direct and indirect subsidiaries. In the opinion of management, the accompanying financial information contains all adjustments, consisting of normal recurring adjustments necessary to present fairly the Company's financial statements as of March 31, 2004 and for the three months ended March 31, 2003 and March 31, 2004.
Reclassifications
Certain reclassifications were made to the prior year financial statements to conform to current year presentation.
3. TRANSACTIONS WITH RELATED PARTIES
The Company has entered into agreements with Whitney and Golden Gate to pay monitoring fees for their services and other expenses. Under the monitoring fee agreements, the Company is obligated to pay an annual aggregate amount of up to $5.0 million, but not less than $2.5 million for an initial period of ten years subject to the provisions of the Credit Agreement as amended. For the three months ended March 31, 2004 and March 31, 2003, the Company expensed monitoring fees in the amount of $1.3 million in both periods and other expenses of $0.5 million and $1.8 million, respectively. As of March 31, 2004, a Whitney affiliated company loaned $5.1 million of the term loan to Herbalife, and another Whitney affiliated company purchased $1.3 million of the senior subordinated notes. Also, in March 2004, Holdings redeemed $17.3 million of the 15.5% Senior Notes held by certain Whitney affiliated companies and paid an additional $5.0 million repurchase premium and $0.5 million accrued interest.
F-47
4. LONG TERM DEBT
Long term debt consisted of the following: (in millions)
| |
December 31, 2003 |
March 31, 2004 |
||||
|---|---|---|---|---|---|---|
| Senior subordinated notes | $ | 158.2 | $ | 158.2 | ||
| Borrowing under senior credit facility | 119.8 | 75.4 | ||||
| 15.5% Senior Notes | 39.6 | — | ||||
| Discount—Senior note warrant | (1.6 | ) | — | |||
| 9.5% Notes | — | 267.5 | ||||
| Capitalized leases | 5.5 | 6.1 | ||||
| Other debt | 3.8 | 3.5 | ||||
| 325.3 | 510.7 | |||||
| Less: current portion | 72.4 | 23.5 | ||||
| $ | 252.9 | $ | 487.2 | |||
In March 2004, the Company and its lenders amended the Credit Agreement. Under the terms of the amendment, the Company made a prepayment of $40.0 million. In connection with this prepayment, the lenders waived the March 31, 2004 mandatory amortization payment of $6.5 million along with a mandatory 50% excess cash flow payment for the year ended December 31, 2003. The Company's debt agreement has a provision that requires the Company to make early payments to the extent of excess cash flow, as defined. The schedule of the principal payments was also modified so that Herbalife was obligated to pay approximately $4.4 million on March 31, 2004 and in each subsequent quarter through June 30, 2008.
In March 2004, Holdings and its wholly-owned subsidiary WH Capital Corporation completed a $275 million offering of 9.5% Notes due 2011 (the "Notes"). The proceeds of the offering together with available cash were used to pay the cash redemption price due upon redemption of all outstanding Holdings convertible preferred shares, including all accrued and unpaid dividends, to redeem Holdings' 15.5% Senior Notes and to pay related fees and expenses. Interest on the Notes will be paid in cash semi-annually in arrear on April 1 and October 1 of each year, starting on October 1, 2004. The Notes are Holdings' general unsecured obligations, ranking equally with any of the existing and future senior indebtedness and senior to all of Holdings' future subordinated indebtedness. Also, the Notes are effectively subordinated to all existing and future indebtedness and other liabilities of Holdings' subsidiaries.
5. CONTINGENCIES
The Company is from time to time engaged in routine litigation. The Company regularly reviews all pending litigation matters in which it is involved and establishes reserves deemed appropriate by management for these litigation matters when a probable loss estimate can be made.
Herbalife is a defendant in a purported class action lawsuit pending in the U.S. District Court of California (Jacobs v. Herbalife International, Inc., et al) originally filed on February 19, 2002 challenging marketing practices of several distributors and Herbalife under various state and federal laws. As a result of recent rulings of the Court, only claims filed under federal securities law remain. The Company understands that the plaintiffs have refilled certain state law claims in the Superior Court of the State of California, County of San Francisco. The Company has not been served with a complaint. The parties are in discussions regarding a possible settlement but no binding settlement agreement has yet been reached. The Company believes that it has meritorious defenses to the suit.
Herbalife and certain of its distributors have been named as defendants in a purported class action lawsuit filed July 16, 2003 in the Circuit Court of Ohio County in the State of West Virginia (Mey v. Herbalife International, Inc., et al). Herbalife removed the lawsuit to federal court and the plaintiff sought to remand the lawsuit to state court. The plaintiff's motion was denied. The complaint alleges that certain telemarketing practices of certain Herbalife distributors violate the Telephone Consumer Protection Act and seek to hold
F-48
Herbalife liable for the practices of its distributors. The Company believes that it has meritorious defenses to the suit.
As a marketer of dietary and nutritional supplements and other products that are ingested by consumers or applied to their bodies, the Company has been subjected to various product liability claims. The effects of these claims to date have not been material to the Company, and the reasonably possible range of exposure on currently existing claims is not material. The Company believes that it has meritorious defenses to the allegations contained in the lawsuits. The Company currently maintains product liability insurance with an annual deductible of $10.0 million.
Certain of the Company's subsidiaries have been subject to tax audits by governmental authorities in their respective countries. In certain of these tax audits, governmental authorities are proposing that significant amounts of additional taxes and related interest and penalties are due. The Company and its tax advisors believe that there are substantial defenses to their allegations that additional taxes are owing, and the Company is vigorously contesting the additional proposed taxes and related charges. These matters may take several years to resolve, and the Company cannot be sure of their ultimate resolution. However, it is the opinion of management that adverse outcomes, if any, will not likely result in a material effect on our financial condition and operating results.
6. COMPREHENSIVE INCOME
Comprehensive income (loss) is summarized as follows: (in millions)
| |
Three Months Ending |
||||||
|---|---|---|---|---|---|---|---|
| |
March 31, 2003 |
March 31, 2004 |
|||||
| Net income (loss) | $ | 16.9 | $ | (0.5 | ) | ||
| Unrealized gain (loss) on derivative instruments | (2.8 | ) | 1.9 | ||||
| Reclassification adjustments for gain (loss) on derivative instruments | 2.3 | (1.1 | ) | ||||
| Foreign currency translation adjustment | 0.5 | (0.1 | ) | ||||
| Comprehensive income (loss) | $ | 16.9 | $ | 0.2 | |||
The net change on derivative instruments represents the fair value changes caused by marking to market these instruments on March 31, 2004. Foreign currency translation adjustment measures the impact of converting primarily foreign currency assets and liabilities of foreign subsidiaries into U.S. dollars.
7. SEGMENT INFORMATION
The Company is a network marketing company that sells a wide range of weight management products, nutritional supplements and personal care products within one reportable segment as defined under SFAS 131, "Disclosures about Segments of an Enterprise and Related Information." The Company's products are primarily manufactured by third party providers and then sold to independent distributors who sell Herbalife products to retail consumers or other distributors.
The Company has operations throughout the world and is organized and managed by geographic area. In the first quarter of 2003, the Company elected to aggregate its operating segments into one reporting segment, as management believes that the operating segments have similar operating characteristics and similar long term operating performance. In making this determination, management believes that the operating segments are similar in the nature of the products sold, the product acquisition process, the types of customers sold to, the methods used to distribute the products, and the nature of the regulatory
F-49
environment. However, the Company does recognize revenue from sales to distributors in four geographic regions: The Americas, Europe, Asia/Pacific Rim and Japan.
| |
Three Months Ended |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
March 31, 2003 |
March 31, 2004 |
||||||
| Net Sales: | ||||||||
| United States | $ | 66.8 | $ | 64.2 | ||||
| Japan | 35.5 | 29.9 | ||||||
| Others | 177.7 | 230.0 | ||||||
| Total Net Sales | $ | 280.0 | $ | 324.1 | ||||
| Operating Margin: | ||||||||
| United States | $ | 29.6 | $ | 27.6 | ||||
| Japan | 17.3 | 15.3 | ||||||
| Others | 76.7 | 101.7 | ||||||
| Total Operating Margin | $ | 123.6 | $ | 144.6 | ||||
Marketing, distribution, and administrative expense |
$ |
84.4 |
$ |
107.8 |
||||
| Interest Expense—net | 9.9 | 27.4 | ||||||
| Income before taxes | 29.3 | 9.4 | ||||||
| Income taxes | 12.4 | 9.9 | ||||||
| Net Income (Loss) | $ | 16.9 | $ | (0.5 | ) | |||
| Net sales by product line: | ||||||||
| Weight management | $ | 119.7 | $ | 142.1 | ||||
| Inner nutrition | 123.1 | 139.3 | ||||||
| Outer Nutrition® | 26.2 | 29.6 | ||||||
| Literature, promotional and other | 11.0 | 13.1 | ||||||
| Total Net Sales | $ | 280.0 | $ | 324.1 | ||||
| Net sales by geographic region: | ||||||||
| The Americas | $ | 100.2 | $ | 111.3 | ||||
| Europe | 105.9 | 136.7 | ||||||
| Asia/Pacific Rim | 38.4 | 46.1 | ||||||
| Japan | 35.5 | 30.0 | ||||||
| Total Net Sales | $ | 280.0 | $ | 324.1 | ||||
| |
December 31, 2003 |
March 31, 2004 |
||||||
| Total Assets: | ||||||||
| United States | $ | 601.0 | $ | 560.0 | ||||
| Japan | 62.9 | 55.2 | ||||||
| Others | 240.1 | 257.8 | ||||||
| Total Assets | $ | 904.0 | $ | 873.0 | ||||
8. POLICY FOR STOCK BASED COMPENSATION
Accounting for Stock Based Compensation. In December 2002, the FASB issued SFAS 148, Accounting for Stock Based Compensation—Transition and Disclosure. SFAS 148 amends SFAS 123, Accounting for Stock Based Compensation, to provide alternative methods of transition for a voluntary change to the fair value based method of accounting for stock based employee compensation. In addition, SFAS 148 amends the disclosure requirements of SFAS 123 to require prominent disclosures in both annual and interim financial
F-50
statements about the method of accounting for stock based employee compensation and the effect of the method used on reported results.
The Company has two stock based employee compensation plans which are the WH Holdings (Cayman Islands) Ltd. Stock Incentive Plan ("The Management Plan") and WH Holdings (Cayman Islands) Ltd. Independent Directors Stock Incentive Plan ("The Independent Directors Plan"). The Management Plan provides for the grant of options to purchase common shares of WH Holdings to members of management of Herbalife following the merger. The Independent Directors Plan provides for the grant of options to purchase common shares of WH Holdings to independent directors of WH Holdings.
The Company applies the intrinsic-value-based method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25, Accounting for Stock Issued to Employees, and related interpretations including FASB Interpretation No. 44, Accounting for Certain Transactions involving Stock Compensation, an interpretation of APB Opinion No. 25, issued in March 2000, to account for its stock option plans. Under this method, compensation expense is recorded on the date of grant only if the then current market price of the underlying stock exceeded the exercise price. SFAS 123 established accounting and disclosure requirements using a fair-value-based method of accounting for stock based employee compensation plans. As allowed by SFAS 123, the Company has elected to continue to apply the intrinsic-value-based method of accounting described above, and has adopted only the disclosure requirements of SFAS 123.
The following table illustrates the effect on net income if the fair-value-based method had been applied to all outstanding and unvested awards in each period:
| |
|
Three Months Ended |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (in millions) |
March 31, 2003 |
March 31, 2004 |
|||||||
| Net income (loss) as reported | $ | 16.9 | $ | (0.5 | ) | ||||
| Add: | Stock-based employee compensation expense included in reported net income | $ | — | $ | 0.3 | ||||
| Deduct: | Stock-based employee compensation expense determined under fair value based methods for all awards | $ | (0.3 | ) | $ | (0.2 | ) | ||
| Pro forma net income | $ | 16.6 | $ | (0.4 | ) | ||||
9. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
The Company designates certain derivatives as fair value hedges. For all qualifying and highly effective fair value hedges, the changes in the fair value of a derivative and the gain or loss on the hedged asset or liability relating to the risk being hedged are recorded currently in earnings. These amounts are recorded in marketing, distribution and administrative expenses and provide offsets to one another.
The Company designates certain derivatives as cash flow hedges. The Company engages in a foreign exchange hedging strategy for which the hedged transactions are forecasted foreign currency denominated intercompany transactions. The hedged risk is the variability of the foreign currency where the hedging strategy involves the purchase and sale of average rate options. For the outstanding cash flow hedges on foreign exchange exposures at March 31, 2004 and March 31, 2003, the maximum length of time over which the Company is hedging these exposures is approximately one year. The Company also engages in an interest rate hedging strategy for which the hedged transactions are forecasted interest payments on the variable rate term loan. The hedged risk is the variability of interest rate where the hedging strategy involves the purchase of interest rate caps. There is no ineffective portion for the three months ended March 31, 2004 and March 31, 2003. For all qualifying and highly effective cash flow hedges, the changes in the effective portion of the fair value of the derivative are recorded in other comprehensive income ("OCI"). At March 31, 2004, the net loss in OCI was $0.6 million. Substantially, all OCI amounts will be reclassified to earnings within 12 months.
F-51
10. RESTRUCTURING RESERVE
As of the date of the Merger, the Company implemented a plan to reduce costs of the business and recorded a severance and restructuring accrual as part of the cost of the Merger. The accrued severance is for identified employees including executives, corporate functions and administrative support.
The following table summarizes the activity in the Company's restructuring accrual: (in millions)
| Balance at December 31, 2003 | $ | 2.5 | ||
| Additional accrual | — | |||
| Cash payments | (0.3 | ) | ||
| Balance at March 31, 2004 | $ | 2.2 | ||
The Company expects to pay these restructuring costs through 2005.
F-52
11. SUPPLEMENTAL INFORMATION
The consolidated financial statement data as of March 31, 2004 and for the three months ended March 31, 2004 and March 31, 2003, respectively have been aggregated by entities that guarantee the Senior Subordinated Notes (the "Guarantors") and entities that do not guarantee the Senior Subordinated Notes (the "Non-Guarantors"). The Guarantors include WH Intermediate, Lux Holdings, Lux Intermediate, Herbalife Lux (collectively, the "Parent Guarantors") and Herbalife's operating subsidiaries in Brazil, Finland, Israel, Japan, Mexico, United Kingdom, U.S. (except for Herbalife Investment Co., LLC), Sweden, Taiwan and Thailand (collectively, the "Subsidiary Guarantors"). All other subsidiaries are Non-Guarantors. Herbalife International, Inc. is the borrower of the Senior Subordinated Notes.
Consolidating condensed unaudited statements of income for guarantors and non-guarantors for the three months ended March 31, 2004 and March 31, 2003 are summarized as follows: (in millions)
| |
Three Months Ended March 31, 2004 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Parent Guarantors |
Herbalife International, Inc. |
Subsidiary Guarantors |
WH Holdings (Cayman Islands) Ltd Non- Guarantor |
Non- Guarantors |
Eliminations |
Total Consolidated |
|||||||||||||||
| Net sales | $ | 150.5 | $ | — | $ | 144.9 | $ | — | $ | 83.9 | $ | (55.2 | ) | $ | 324.1 | |||||||
Cost of sales |
30.6 |
— |
44.3 |
— |
43.1 |
(54.4 |
) |
63.6 |
||||||||||||||
| Royalty overrides | 4.2 | — | 63.2 | — | 48.5 | — | 115.9 | |||||||||||||||
| Marketing, distribution & administrative expenses | 3.3 | 7.2 | 77.0 | (0.1 | ) | 20.4 | — | 107.8 | ||||||||||||||
| Equity in subsidiary (income) loss | (2.1 | ) | 0.7 | (0.8 | ) | (17.3 | ) | — | 19.5 | — | ||||||||||||
| Interest expense—net | 0.2 | 8.5 | 1.9 | 18.2 | (1.4 | ) | — | 27.4 | ||||||||||||||
| Intercompany charges | 97.0 | (21.9 | ) | (37.0 | ) | — | (38.1 | ) | — | — | ||||||||||||
| Income before income taxes | 17.3 | 5.5 | (3.7 | ) | (0.8 | ) | 11.4 | (20.3 | ) | 9.4 | ||||||||||||
| Income tax expense (benefit) | — | 3.4 | 2.7 | — | 3.8 | — | 9.9 | |||||||||||||||
| NET INCOME (LOSS) | $ | 17.3 | $ | 2.1 | $ | (6.4 | ) | $ | (0.8 | ) | $ | 7.6 | $ | (20.3 | ) | $ | (0.5 | ) | ||||
| |
Three Months Ended March 31, 2003 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Parent Guarantors |
Herbalife International, Inc. |
Subsidiary Guarantors |
WH Holdings (Cayman Islands) Ltd Non- Guarantor |
Non- Guarantors |
Eliminations |
Total Consolidated |
||||||||||||||
| Net sales | $ | — | $ | — | $ | 246.5 | $ | — | $ | 60.4 | $ | (26.9 | ) | $ | 280.0 | ||||||
| Cost of sales | — | — | 55.0 | — | 28.7 | (26.7 | ) | 57.0 | |||||||||||||
| Royalty overrides | — | — | 63.1 | — | 36.4 | — | 99.5 | ||||||||||||||
| Marketing, distribution & administrative expenses | — | 2.4 | 63.4 | 0.1 | 18.5 | — | 84.4 | ||||||||||||||
| Equity in subsidiary (income) loss | (18.6 | ) | (24.3 | ) | (0.4 | ) | (18.6 | ) | — | 61.9 | — | ||||||||||
| Interest expense—net | — | 8.6 | (0.3 | ) | 1.6 | — | — | 9.9 | |||||||||||||
| Intercompany charges | — | (2.2 | ) | 31.6 | — | (29.4 | ) | — | — | ||||||||||||
| Income before income taxes | 18.6 | 15.5 | 34.1 | 16.9 | 6.2 | (62.1 | ) | 29.2 | |||||||||||||
| Income tax expense (benefit) | — | (3.1 | ) | 13.5 | — | 1.9 | — | 12.3 | |||||||||||||
| NET INCOME (LOSS) | $ | 18.6 | $ | 18.6 | $ | 20.6 | $ | 16.9 | $ | 4.3 | $ | (62.1 | ) | $ | 16.9 | ||||||
F-53
Consolidating condensed balance sheet data for guarantors and non-guarantors as of March 31, 2004 and December 31, 2003 are summarized as follows: (in millions)
| |
March 31, 2004 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Parent Guarantors |
Herbalife International, Inc. |
Subsidiary Guarantors |
WH Holdings (Cayman Islands) Ltd Non- Guarantor |
Non- Guarantors |
Eliminations |
Total Consolidated |
|||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||||||
| Cash and marketable securities | $ | 9.9 | $ | 0.1 | $ | 64.5 | $ | 2.7 | $ | 45.8 | $ | — | $ | 123.0 | ||||||||
| Receivables | — | 3.1 | 23.1 | (0.2 | ) | 11.0 | (3.2 | ) | 33.8 | |||||||||||||
| Intercompany receivables | (6.4 | ) | 162.4 | (73.6 | ) | — | (82.4 | ) | — | — | ||||||||||||
| Inventories | 27.4 | — | 26.4 | — | 16.9 | (6.6 | ) | 64.1 | ||||||||||||||
| Other current assets | 8.0 | 1.4 | 19.8 | — | 4.2 | — | 33.4 | |||||||||||||||
| Total current assets | 38.9 | 167.0 | 60.2 | 2.5 | (4.5 | ) | (9.8 | ) | 254.3 | |||||||||||||
| PROPERTY, NET | 1.9 | 0.4 | 38.4 | — | 5.0 | — | 45.7 | |||||||||||||||
| OTHER NON-CURRENT ASSETS | 67.9 | 436.2 | 130.8 | 256.5 | 68.6 | (387.0 | ) | 573.0 | ||||||||||||||
| TOTAL ASSETS | $ | 108.7 | $ | 603.6 | $ | 229.4 | $ | 259.0 | $ | 69.1 | $ | (396.8 | ) | $ | 873.0 | |||||||
| CURRENT LIABILITIES: | ||||||||||||||||||||||
| Accounts payable | $ | 8.3 | $ | — | $ | 9.9 | — | $ | 5.0 | $ | — | $ | 23.2 | |||||||||
| Royalties overrides | (0.9 | ) | — | 40.6 | — | 28.8 | — | 68.5 | ||||||||||||||
| Accrued compensation and expenses | 13.4 | 3.9 | 46.8 | 1.6 | 15.4 | — | 81.1 | |||||||||||||||
| Other current liabilities | 3.7 | 2.5 | 55.2 | — | 4.4 | (3.1 | ) | 62.7 | ||||||||||||||
| Total current liabilities | 24.5 | 6.4 | 152.5 | 1.6 | 53.6 | (3.1 | ) | 235.5 | ||||||||||||||
| NON-CURRENT LIABILITIES | (0.2 | ) | 353.3 | (0.6 | ) | 267.5 | 0.7 | — | 620.7 | |||||||||||||
| STOCKHOLDER'S EQUITY | 84.4 | 243.9 | 77.5 | (10.1 | ) | 14.8 | (393.7 | ) | 16.8 | |||||||||||||
| TOTAL LIABILITIES & STOCKHOLDER'S EQUITY | $ | 108.7 | $ | 603.6 | $ | 229.4 | 259.0 | $ | 69.1 | $ | (396.8 | ) | $ | 873.0 | ||||||||
| |
December 31, 2003 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Parent Guarantors |
Herbalife International, Inc. |
Subsidiary Guarantors |
WH Holdings (Cayman Islands) Ltd Non- Guarantor |
Non- Guarantors |
Eliminations |
Total Consolidated |
|||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||||||
| Cash and marketable securities | $ | 13.8 | $ | 0.1 | $ | 92.5 | $ | 9.4 | $ | 40.6 | $ | — | $ | 156.4 | ||||||||
| Receivables | — | — | 23.0 | 1.5 | 7.5 | — | 32.0 | |||||||||||||||
| Intercompany receivables | (23.3 | ) | 196.7 | (89.4 | ) | — | (84.0 | ) | — | — | ||||||||||||
| Inventories | 26.0 | — | 23.9 | — | 15.0 | (5.5 | ) | 59.4 | ||||||||||||||
| Other current assets | 2.2 | (2.5 | ) | 26.9 | — | 3.4 | — | 30.0 | ||||||||||||||
| Total current assets | 18.7 | 194.3 | 76.9 | 10.9 | (17.5 | ) | (5.5 | ) | 277.8 | |||||||||||||
| Property, net | 2.1 | 0.3 | 37.7 | — | 5.3 | — | 45.4 | |||||||||||||||
| OTHER NON-CURRENT ASSETS | 65.8 | 448.9 | 129.8 | 238.7 | 68.5 | (370.9 | ) | 580.8 | ||||||||||||||
| TOTAL ASSETS | $ | 86.6 | $ | 643.5 | $ | 244.4 | 249.6 | $ | 56.3 | $ | (376.4 | ) | $ | 904.0 | ||||||||
| CURRENT LIABILITIES: | ||||||||||||||||||||||
| Accounts payable | $ | 8.2 | $ | — | $ | 10.4 | $ | 0.1 | $ | 3.8 | $ | — | $ | 22.5 | ||||||||
| Royalties overrides | 0.7 | — | 45.7 | — | 30.1 | — | 76.5 | |||||||||||||||
| Accrued compensation and expenses | 10.2 | 8.7 | 44.7 | — | 15.2 | — | 78.8 | |||||||||||||||
| Other current liabilities | 0.4 | 41.1 | 55.6 | (0.2 | ) | 1.5 | — | 98.4 | ||||||||||||||
| Total current liabilities | 19.5 | 49.8 | 156.4 | (0.1 | ) | 50.6 | — | 276.2 | ||||||||||||||
| NON-CURRENT LIABILITIES | 0.3 | 351.9 | (0.9 | ) | 38.0 | 0.7 | — | 390.0 | ||||||||||||||
| STOCKHOLDER'S EQUITY | 66.8 | 241.8 | 88.9 | 211.7 | 5.0 | (376.4 | ) | 237.8 | ||||||||||||||
| TOTAL LIABILITIES & STOCKHOLDER'S EQUITY | $ | 86.6 | $ | 643.5 | $ | 244.4 | 249.6 | $ | 56.3 | $ | (376.4 | ) | $ | 904.0 | ||||||||
F-54
Consolidating condensed statement of cash flows data for guarantors and non-guarantors for the three months ended March 31 2004 and March 31, 2003 is summarized as follows: (in millions)
| |
Three Months Ended March 31, 2004 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Parent Guarantors |
Herbalife International, Inc. |
Subsidiary Guarantors |
WH Holdings (Cayman Islands) Ltd Non- Guarantor |
Non- Guarantors |
Eliminations |
Total Consolidated |
|||||||||||||||
| Net cash provided by (used in) operating activities | $ | (1.7 | ) | $ | 39.5 | $ | (7.3 | ) | $ | 18.2 | $ | 7.5 | $ | (30.5 | ) | $ | 25.7 | |||||
| Net cash provided by (used in) investing activities | (0.8 | ) | 4.7 | (15.5 | ) | (14.1 | ) | (3.1 | ) | 27.3 | (1.5 | ) | ||||||||||
| Net cash provided by (used in) financing activities | — | (44.3 | ) | (5.5 | ) | (5.0 | ) | 1.4 | 3.2 | (50.2 | ) | |||||||||||
| Effect of exchange rate changes on cash | (1.3 | ) | — | 0.3 | (0.1 | ) | (0.6 | ) | — | (1.7 | ) | |||||||||||
| Cash at beginning of period | 13.7 | 0.2 | 92.5 | 3.7 | 40.6 | — | 150.7 | |||||||||||||||
| Cash at end of period | $ | 9.9 | $ | 0.1 | $ | 64.5 | $ | 2.7 | $ | 45.8 | $ | — | $ | 123.0 | ||||||||
| |
Three Months Ended March 31, 2003 |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Parent Guarantors |
Herbalife International, Inc. |
Subsidiary Guarantors |
WH Holdings (Cayman Islands) Ltd Non- Guarantor |
Non- Guarantors |
Eliminations |
Total Consolidated |
|||||||||||||||
| Net cash provided by (used in) operating activities | $ | 18.6 | $ | 19.6 | $ | (0.8 | ) | $ | 16.7 | $ | 10.7 | $ | (68.3 | ) | $ | (3.5 | ) | |||||
| Net cash provided by (used in) investing activities | (18.6 | ) | (19.8 | ) | 3.0 | (17.0 | ) | 0.3 | 63.2 | 11.1 | ||||||||||||
| Net cash provided by (used in) financing activities | — | — | (5.6 | ) | 0.3 | (1.6 | ) | 5.1 | (1.8 | ) | ||||||||||||
| Effect of exchange rate changes on cash | — | — | (0.1 | ) | — | 0.2 | — | 0.1 | ||||||||||||||
| Cash at beginning of period | — | 0.5 | 38.2 | — | 25.5 | — | 64.2 | |||||||||||||||
| Cash at end of period | $ | — | $ | 0.3 | $ | 34.7 | $ | — | $ | 35.1 | $ | — | $ | 70.1 | ||||||||
F-55
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not rely on any unauthorized information or representations. This prospectus does not offer to sell or ask for offers to buy any securities other than those to which this prospectus relates and it does not constitute an offer to sell or ask for offers to buy any of the securities in any jurisdiction where it is unlawful, where the person making the offer is not qualified to do so, or to any person who cannot legally be offered the securities. The information contained in this prospectus is current only as of its date.
Until September 7, 2004, all dealers that effect transactions in these securities, whether or not participating in this exchange offer, may be required to deliver a prospectus. Each broker-dealer that receives Notes for its own account pursuant to the exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of such Notes. For a period of 90 days after the Expiration Date of the exchange offer this prospectus will be made available to any broker-dealer for use in connection with any such resale.
WH HOLDINGS (CAYMAN ISLANDS) LTD.
WH CAPITAL CORPORATION
Indirect Parent of
![]()
Exchange Offer for All Outstanding
91/2% Notes Due April 1, 2011
(CUSIP Nos. 92926X AA 3 and G96013 AA 8)
For New
91/2% Notes Due April 1, 2011
Which Have Been Registered
Under the Securities Act of 1933
PROSPECTUS
June 9, 2004